Real-world mepolizumab in the prospective severe asthma REALITI-A study: initial analysis
- PMID: 32817259
- PMCID: PMC7559868
- DOI: 10.1183/13993003.00151-2020
Real-world mepolizumab in the prospective severe asthma REALITI-A study: initial analysis
Abstract
Introduction: Efficacy of mepolizumab, an anti-interleukin-5 monoclonal antibody, was demonstrated in randomised controlled trials; data on its real-world impact in routine clinical practice are starting to emerge. We assessed the effectiveness and safety of mepolizumab prescribed for patients in the real world.
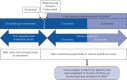
Methods: REALITI-A is a global, prospective, observational cohort study, collecting data from routine healthcare visits from patients with asthma. Patients newly prescribed mepolizumab for severe asthma with 12 months of relevant medical history pre-mepolizumab (collected retrospectively) were enrolled. An initial analysis of data from early initiators who had completed 1 year of follow-up (as of February 28, 2019) was conducted. The primary objective was to compare the rate of clinically significant exacerbations (requiring oral corticosteroids (OCS) and/or hospitalisation and/or emergency department visit) before and after mepolizumab; exacerbations requiring hospitalisation and/or emergency department visit and change in maintenance OCS use were secondary objectives. Treatment-related adverse events were reported.
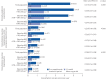
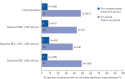
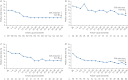
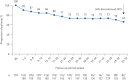
Results: Overall, 368 mepolizumab-treated patients were included. Rates of clinically significant exacerbations were reduced by 69% from 4.63 per person per year pre-treatment to 1.43 per person per year during follow-up (p<0.001), as were those requiring hospitalisation and/or emergency department visit (from 1.14 to 0.27 per person per year; 77% reduction). In 159 patients with maintenance OCS dose data available during the pre-treatment period, median daily dose decreased from 10.0 (pre-treatment) to 5.0 mg·day-1 by week 21-24 of follow-up, sustained until week 53-56. No new safety signals were reported.
Conclusion: These data demonstrate that the effectiveness of mepolizumab is consistent with clinical trial results under real-world settings, with significant reductions in exacerbations and daily maintenance OCS dose.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: T. Harrison reports that the study and writing support was funded by GSK, and received personal fees for lectures and advisory board work from GSK and AstraZeneca, personal fees for advisory board work from Vectura, outside the submitted work. Conflict of interest: G.W. Canonica reports that the study and writing support was funded by GSK, and received grants and personal fees for lectures and advisory board work from GSK, AstraZeneca, Sanofi-Genzyme, Regeneron and Novartis, outside the submitted work. Conflict of interest: G. Chupp reports that the study and writing support was funded by GSK, and received grants and personal fees for lectures and advisory board work from GSK, AstraZeneca, Genentech, Sanofi-Genzyme, Regeneron, Teva and Novartis, outside the submitted work. Conflict of interest: J. Lee reports that the study and writing support was funded by GSK, and received grants from Regeneron, Genentech, Roche and Takeda, grants and personal fees for lectures from GSK, Sanofi-Genzyme, Novartis, Medexus and AstraZeneca, personal fees for lectures from Mylan, Aralez and Merck, outside the submitted work. Conflict of interest: F. Schleich reports that the study and writing support was funded by GSK, and received grants and personal fees for lectures and advisory board work from AstraZeneca, grants, personal fees for lectures and advisory board work, and nonfinancial support for travel from Chiesi and Novartis, personal fees for lectures from Menarini and Mundipharma, grants and personal fees for lectures, consultancy and advisory board work from GSK, outside the submitted work. Conflict of interest: T. Welte reports that the study and writing support was funded by GSK, and received grants and personal fees for lectures and advisory board work from AstraZeneca, personal fees for lectures and advisory board work from Novartis and Sanofi, personal fees for advisory board work from GSK, outside the submitted work. Conflict of interest: A. Valero reports that the study and writing support was funded by GSK, and received personal fees for consultancy and lectures from AstraZeneca, Novartis and Mundipharma, personal fees for consultancy from Sanofi and Boehringer, personal fees for lectures from Chiesi and GSK, outside the submitted work. Conflict of interest: K. Gemzoe reports that the study and writing support was funded by GSK, and is an employee of and holds shares/options in GSK. Conflict of interest: A. Maxwell reports that the study and writing support was funded by GSK, and is an employee of and holds shares/options in GSK. Conflict of interest: S. Joksaite reports that the study and writing support was funded by GSK, and is an employee of and holds shares/options in GSK. Conflict of interest: S. Yang reports that the study and writing support was funded by GSK, and is an employee of and holds shares/options in GSK. Conflict of interest: P. Howarth reports that the study and writing support was funded by GSK, and is an employee of and holds shares/options in GSK. Conflict of interest: M.K. Van Dyke reports that the study and writing support was funded by GSK, and is an employee of and holds shares/options in GSK.
Figures
References
-
- The Global Asthma Network. The Global Asthma Report 2018 2018. www.globalasthmareport.org/Global%20Asthma%20Report%202018.pdf Date last accessed: September 14, 2019.
