Iron-responsive-like elements and neurodegenerative ferroptosis
- PMID: 32817306
- PMCID: PMC7433652
- DOI: 10.1101/lm.052282.120
Iron-responsive-like elements and neurodegenerative ferroptosis
Abstract
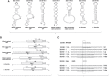
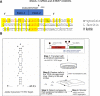
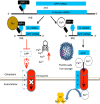
A set of common-acting iron-responsive 5'untranslated region (5'UTR) motifs can fold into RNA stem loops that appear significant to the biology of cognitive declines of Parkinson's disease dementia (PDD), Lewy body dementia (LDD), and Alzheimer's disease (AD). Neurodegenerative diseases exhibit perturbations of iron homeostasis in defined brain subregions over characteristic time intervals of progression. While misfolding of Aβ from the amyloid-precursor-protein (APP), alpha-synuclein, prion protein (PrP) each cause neuropathic protein inclusions in the brain subregions, iron-responsive-like element (IRE-like) RNA stem-loops reside in their transcripts. APP and αsyn have a role in iron transport while gene duplications elevate the expression of their products to cause rare familial cases of AD and PDD. Of note, IRE-like sequences are responsive to excesses of brain iron in a potential feedback loop to accelerate neuronal ferroptosis and cognitive declines as well as amyloidosis. This pathogenic feedback is consistent with the translational control of the iron storage protein ferritin. We discuss how the IRE-like RNA motifs in the 5'UTRs of APP, alpha-synuclein and PrP mRNAs represent uniquely folded drug targets for therapies to prevent perturbed iron homeostasis that accelerates AD, PD, PD dementia (PDD) and Lewy body dementia, thus preventing cognitive deficits. Inhibition of alpha-synuclein translation is an option to block manganese toxicity associated with early childhood cognitive problems and manganism while Pb toxicity is epigenetically associated with attention deficit and later-stage AD. Pathologies of heavy metal toxicity centered on an embargo of iron export may be treated with activators of APP and ferritin and inhibitors of alpha-synuclein translation.
© 2020 Rogers and Cahill; Published by Cold Spring Harbor Laboratory Press.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
