HIV-1 Gag protein with or without p6 specifically dimerizes on the viral RNA packaging signal
- PMID: 32817318
- PMCID: PMC7573273
- DOI: 10.1074/jbc.RA120.014835
HIV-1 Gag protein with or without p6 specifically dimerizes on the viral RNA packaging signal
Abstract
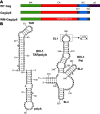
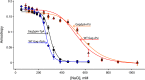
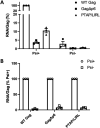
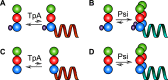
The HIV-1 Gag protein is responsible for genomic RNA (gRNA) packaging and immature viral particle assembly. Although the presence of gRNA in virions is required for viral infectivity, in its absence, Gag can assemble around cellular RNAs and form particles resembling gRNA-containing particles. When gRNA is expressed, it is selectively packaged despite the presence of excess host RNA, but how it is selectively packaged is not understood. Specific recognition of a gRNA packaging signal (Psi) has been proposed to stimulate the efficient nucleation of viral assembly. However, the heterogeneity of Gag-RNA interactions renders capturing this transient nucleation complex using traditional structural biology approaches challenging. Here, we used native MS to investigate RNA binding of wild-type (WT) Gag and Gag lacking the p6 domain (GagΔp6). Both proteins bind to Psi RNA primarily as dimers, but to a control RNA primarily as monomers. The dimeric complexes on Psi RNA require an intact dimer interface within Gag. GagΔp6 binds to Psi RNA with high specificity in vitro and also selectively packages gRNA in particles produced in mammalian cells. These studies provide direct support for the idea that Gag binding to Psi specifically promotes nucleation of Gag-Gag interactions at the early stages of immature viral particle assembly in a p6-independent manner.
Keywords: Gag; Gag p6 domain; HIV; MS; RNA; RNA binding protein; RNA-protein interaction; genomic RNA packaging; human immunodeficiency virus; mass spectrometry; native mass spectrometry; virus assembly.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

