Phosphorylated CtIP bridges DNA to promote annealing of broken ends
- PMID: 32817418
- PMCID: PMC7474685
- DOI: 10.1073/pnas.2008645117
Phosphorylated CtIP bridges DNA to promote annealing of broken ends
Abstract
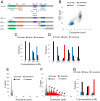
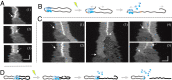
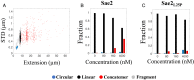
The early steps of DNA double-strand break (DSB) repair in human cells involve the MRE11-RAD50-NBS1 (MRN) complex and its cofactor, phosphorylated CtIP. The roles of these proteins in nucleolytic DSB resection are well characterized, but their role in bridging the DNA ends for efficient and correct repair is much less explored. Here we study the binding of phosphorylated CtIP, which promotes the endonuclease activity of MRN, to single long (∼50 kb) DNA molecules using nanofluidic channels and compare it to the yeast homolog Sae2. CtIP bridges DNA in a manner that depends on the oligomeric state of the protein, and truncated mutants demonstrate that the bridging depends on CtIP regions distinct from those that stimulate the nuclease activity of MRN. Sae2 is a much smaller protein than CtIP, and its bridging is significantly less efficient. Our results demonstrate that the nuclease cofactor and structural functions of CtIP may depend on the same protein population, which may be crucial for CtIP functions in both homologous recombination and microhomology-mediated end-joining.
Keywords: CtIP; DNA repair; homologous recombination; nanofluidics; single DNA molecule biophysics.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Ranjha L., Howard S. M., Cejka P., Main steps in DNA double-strand break repair: An introduction to homologous recombination and related processes. Chromosoma 127, 187–214 (2018). - PubMed
-
- Miller H., Zhou Z., Shepherd J., Wollman A. J. M., Leake M. C., Single-molecule techniques in biophysics: A review of the progress in methods and applications. Rep. Prog. Phys. 81, 024601 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

