Synthetic conversion of leaf chloroplasts into carotenoid-rich plastids reveals mechanistic basis of natural chromoplast development
- PMID: 32817419
- PMCID: PMC7474630
- DOI: 10.1073/pnas.2004405117
Synthetic conversion of leaf chloroplasts into carotenoid-rich plastids reveals mechanistic basis of natural chromoplast development
Abstract
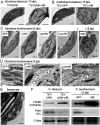
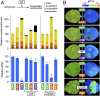
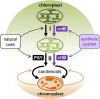
Plastids, the defining organelles of plant cells, undergo physiological and morphological changes to fulfill distinct biological functions. In particular, the differentiation of chloroplasts into chromoplasts results in an enhanced storage capacity for carotenoids with industrial and nutritional value such as beta-carotene (provitamin A). Here, we show that synthetically inducing a burst in the production of phytoene, the first committed intermediate of the carotenoid pathway, elicits an artificial chloroplast-to-chromoplast differentiation in leaves. Phytoene overproduction initially interferes with photosynthesis, acting as a metabolic threshold switch mechanism that weakens chloroplast identity. In a second stage, phytoene conversion into downstream carotenoids is required for the differentiation of chromoplasts, a process that involves a concurrent reprogramming of nuclear gene expression and plastid morphology for improved carotenoid storage. We hence demonstrate that loss of photosynthetic competence and enhanced production of carotenoids are not just consequences but requirements for chloroplasts to differentiate into chromoplasts.
Keywords: carotenoid; chromoplast; differentiation; phytoene; synthetic.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
Chloroplast-to-chromoplast transition envisions provitamin A biofortification in green vegetables.Plant Cell Rep. 2021 May;40(5):799-804. doi: 10.1007/s00299-021-02684-7. Epub 2021 Mar 23. Plant Cell Rep. 2021. PMID: 33754204
-
Novel insights into the contribution of plastoglobules and reactive oxygen species to chromoplast differentiation.New Phytol. 2023 Mar;237(5):1696-1710. doi: 10.1111/nph.18585. Epub 2022 Dec 5. New Phytol. 2023. PMID: 36307969
-
ORHis, a Natural Variant of OR, Specifically Interacts with Plastid Division Factor ARC3 to Regulate Chromoplast Number and Carotenoid Accumulation.Mol Plant. 2020 Jun 1;13(6):864-878. doi: 10.1016/j.molp.2020.03.007. Epub 2020 Mar 25. Mol Plant. 2020. PMID: 32222485
-
Research progress on differentiation and regulation of plant chromoplasts.Mol Biol Rep. 2024 Jul 13;51(1):810. doi: 10.1007/s11033-024-09753-6. Mol Biol Rep. 2024. PMID: 39001942 Review.
-
Differentiation of chromoplasts and other plastids in plants.Plant Cell Rep. 2019 Jul;38(7):803-818. doi: 10.1007/s00299-019-02420-2. Epub 2019 May 11. Plant Cell Rep. 2019. PMID: 31079194 Free PMC article. Review.
Cited by
-
A Small Subunit of Geranylgeranyl Diphosphate Synthase Functions as an Active Regulator of Carotenoid Synthesis in Nicotiana tabacum.Int J Mol Sci. 2023 Jan 4;24(2):992. doi: 10.3390/ijms24020992. Int J Mol Sci. 2023. PMID: 36674507 Free PMC article.
-
"Omics" insights into plastid behavior toward improved carotenoid accumulation.Front Plant Sci. 2022 Oct 6;13:1001756. doi: 10.3389/fpls.2022.1001756. eCollection 2022. Front Plant Sci. 2022. PMID: 36275568 Free PMC article. Review.
-
Characterization of BoaCRTISO Reveals Its Role in Carotenoid Biosynthesis in Chinese Kale.Front Plant Sci. 2021 May 14;12:662684. doi: 10.3389/fpls.2021.662684. eCollection 2021. Front Plant Sci. 2021. PMID: 34054903 Free PMC article.
-
Comparative transcriptome analyses shed light on carotenoid production and plastid development in melon fruit.Hortic Res. 2021 May 1;8(1):112. doi: 10.1038/s41438-021-00547-6. Hortic Res. 2021. PMID: 33931604 Free PMC article.
-
RNA Viral Vectors for Accelerating Plant Synthetic Biology.Front Plant Sci. 2021 Jun 23;12:668580. doi: 10.3389/fpls.2021.668580. eCollection 2021. Front Plant Sci. 2021. PMID: 34249040 Free PMC article.
References
-
- Jarvis P., López-Juez E., Biogenesis and homeostasis of chloroplasts and other plastids. Nat. Rev. Mol. Cell Biol. 14, 787–802 (2013). - PubMed
-
- Egea I. et al. ., Chromoplast differentiation: Current status and perspectives. Plant Cell Physiol. 51, 1601–1611 (2010). - PubMed
-
- Rodriguez-Concepcion M. et al. ., A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 70, 62–93 (2018). - PubMed
-
- Sun T. et al. ., Carotenoid metabolism in plants: The role of plastids. Mol. Plant 11, 58–74 (2018). - PubMed

