Structural elucidation of the cis-prenyltransferase NgBR/DHDDS complex reveals insights in regulation of protein glycosylation
- PMID: 32817466
- PMCID: PMC7456142
- DOI: 10.1073/pnas.2008381117
Structural elucidation of the cis-prenyltransferase NgBR/DHDDS complex reveals insights in regulation of protein glycosylation
Abstract
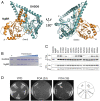
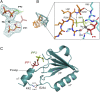
Cis-prenyltransferase (cis-PTase) catalyzes the rate-limiting step in the synthesis of glycosyl carrier lipids required for protein glycosylation in the lumen of endoplasmic reticulum. Here, we report the crystal structure of the human NgBR/DHDDS complex, which represents an atomic resolution structure for any heterodimeric cis-PTase. The crystal structure sheds light on how NgBR stabilizes DHDDS through dimerization, participates in the enzyme's active site through its C-terminal -RXG- motif, and how phospholipids markedly stimulate cis-PTase activity. Comparison of NgBR/DHDDS with homodimeric cis-PTase structures leads to a model where the elongating isoprene chain extends beyond the enzyme's active site tunnel, and an insert within the α3 helix helps to stabilize this energetically unfavorable state to enable long-chain synthesis to occur. These data provide unique insights into how heterodimeric cis-PTases have evolved from their ancestral, homodimeric forms to fulfill their function in long-chain polyprenol synthesis.
Keywords: cis-prenyltransferase; dolichol; glycosylation.
Conflict of interest statement
The authors declare no competing interest.
Figures




Similar articles
-
A conserved C-terminal RXG motif in the NgBR subunit of cis-prenyltransferase is critical for prenyltransferase activity.J Biol Chem. 2017 Oct 20;292(42):17351-17361. doi: 10.1074/jbc.M117.806034. Epub 2017 Aug 23. J Biol Chem. 2017. PMID: 28842490 Free PMC article.
-
Structural basis of heterotetrameric assembly and disease mutations in the human cis-prenyltransferase complex.Nat Commun. 2020 Oct 19;11(1):5273. doi: 10.1038/s41467-020-18970-z. Nat Commun. 2020. PMID: 33077723 Free PMC article.
-
Mutation of Nogo-B receptor, a subunit of cis-prenyltransferase, causes a congenital disorder of glycosylation.Cell Metab. 2014 Sep 2;20(3):448-57. doi: 10.1016/j.cmet.2014.06.016. Epub 2014 Jul 24. Cell Metab. 2014. PMID: 25066056 Free PMC article.
-
Molecular analysis of cis-prenyl chain elongating enzymes.Nat Prod Rep. 2003 Feb;20(1):111-8. doi: 10.1039/b108934j. Nat Prod Rep. 2003. PMID: 12636086 Review.
-
How to Implement Digital Clinical Consultations in UK Maternity Care: the ARM@DA Realist Review.Health Soc Care Deliv Res. 2025 May;13(22):1-77. doi: 10.3310/WQFV7425. Health Soc Care Deliv Res. 2025. PMID: 40417997 Review.
Cited by
-
DHDDS and NUS1: A Converging Pathway and Common Phenotype.Mov Disord Clin Pract. 2024 Jan;11(1):76-85. doi: 10.1002/mdc3.13920. Epub 2023 Nov 28. Mov Disord Clin Pract. 2024. PMID: 38291835 Free PMC article. Review.
-
Case Report: Clinical Features of a Chinese Boy With Epileptic Seizures and Intellectual Disabilities Who Carries a Truncated NUS1 Variant.Front Pediatr. 2021 Aug 31;9:725231. doi: 10.3389/fped.2021.725231. eCollection 2021. Front Pediatr. 2021. PMID: 34532305 Free PMC article.
-
Loss of cis-PTase function in the liver promotes a highly penetrant form of fatty liver disease that rapidly transitions to hepatocellular carcinoma.bioRxiv [Preprint]. 2023 Nov 15:2023.11.13.566870. doi: 10.1101/2023.11.13.566870. bioRxiv. 2023. PMID: 38014178 Free PMC article. Preprint.
-
Adult-onset rapidly worsening progressive myoclonic epilepsy caused by a novel variant in DHDDS.Ann Clin Transl Neurol. 2021 Dec;8(12):2319-2326. doi: 10.1002/acn3.51483. Epub 2021 Nov 27. Ann Clin Transl Neurol. 2021. PMID: 34837344 Free PMC article.
-
Perspectives on Retinal Dolichol Metabolism, and Visual Deficits in Dolichol Metabolism-Associated Inherited Disorders.Adv Exp Med Biol. 2023;1415:449-456. doi: 10.1007/978-3-031-27681-1_66. Adv Exp Med Biol. 2023. PMID: 37440071 Review.
References
-
- Takahashi S., Koyama T., Structure and function of cis-prenyl chain elongating enzymes. Chem. Rec. 6, 194–205 (2006). - PubMed
-
- Shimizu N., Koyama T., Ogura K., Molecular cloning, expression, and purification of undecaprenyl diphosphate synthase. No sequence similarity between E- and Z-prenyl diphosphate synthases. J. Biol. Chem. 273, 19476–19481 (1998). - PubMed
-
- Endo S., Zhang Y. W., Takahashi S., Koyama T., Identification of human dehydrodolichyl diphosphate synthase gene. Biochim. Biophys. Acta 1625, 291–295 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

