Selenoprotein N is an endoplasmic reticulum calcium sensor that links luminal calcium levels to a redox activity
- PMID: 32817544
- PMCID: PMC7474598
- DOI: 10.1073/pnas.2003847117
Selenoprotein N is an endoplasmic reticulum calcium sensor that links luminal calcium levels to a redox activity
Abstract
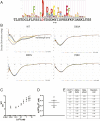
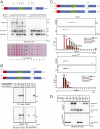
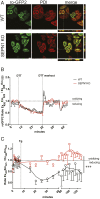
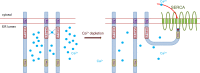
The endoplasmic reticulum (ER) is the reservoir for calcium in cells. Luminal calcium levels are determined by calcium-sensing proteins that trigger calcium dynamics in response to calcium fluctuations. Here we report that Selenoprotein N (SEPN1) is a type II transmembrane protein that senses ER calcium fluctuations by binding this ion through a luminal EF-hand domain. In vitro and in vivo experiments show that via this domain, SEPN1 responds to diminished luminal calcium levels, dynamically changing its oligomeric state and enhancing its redox-dependent interaction with cellular partners, including the ER calcium pump sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA). Importantly, single amino acid substitutions in the EF-hand domain of SEPN1 identified as clinical variations are shown to impair its calcium-binding and calcium-dependent structural changes, suggesting a key role of the EF-hand domain in SEPN1 function. In conclusion, SEPN1 is a ER calcium sensor that responds to luminal calcium depletion, changing its oligomeric state and acting as a reductase to refill ER calcium stores.
Keywords: SEPN1; calcium sensor; endoplasmic reticulum; stress of the endoplasmic reticulum.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Berridge M. J., Lipp P., Bootman M. D., The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 (2000). - PubMed
-
- Boncompagni S., Pozzer D., Viscomi C., Ferreiro A., Zito E., Physical and functional cross-talk between endo-sarcoplasmic reticulum and mitochondria in skeletal muscle. Antioxid. Redox Signal. 32, 873–883 (2020). - PubMed
-
- Burdakov D., Petersen O. H., Verkhratsky A., Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium 38, 303–310 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

