Urine DNA methylation assay enables early detection and recurrence monitoring for bladder cancer
- PMID: 32817589
- PMCID: PMC7685755
- DOI: 10.1172/JCI139597
Urine DNA methylation assay enables early detection and recurrence monitoring for bladder cancer
Abstract
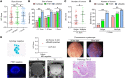
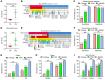
BACKGROUNDCurrent methods for the detection and surveillance of bladder cancer (BCa) are often invasive and/or possess suboptimal sensitivity and specificity, especially in early-stage, minimal, and residual tumors.METHODSWe developed an efficient method, termed utMeMA, for the detection of urine tumor DNA methylation at multiple genomic regions by MassARRAY. We identified the BCa-specific methylation markers by combined analyses of cohorts from Sun Yat-sen Memorial Hospital (SYSMH), The Cancer Genome Atlas (TCGA), and the Gene Expression Omnibus (GEO) database. The BCa diagnostic model was built in a retrospective cohort (n = 313) and validated in a multicenter, prospective cohort (n = 175). The performance of this diagnostic assay was analyzed and compared with urine cytology and FISH.RESULTSWe first discovered 26 significant methylation markers of BCa in combined analyses. We built and validated a 2-marker-based diagnostic model that discriminated among patients with BCa with high accuracy (86.7%), sensitivity (90.0%), and specificity (83.1%). Furthermore, the utMeMA-based assay achieved a great improvement in sensitivity over urine cytology and FISH, especially in the detection of early-stage (stage Ta and low-grade tumor, 64.5% vs. 11.8%, 15.8%), minimal (81.0% vs. 14.8%, 37.9%), residual (93.3% vs. 27.3%, 64.3%), and recurrent (89.5% vs. 31.4%, 52.8%) tumors. The urine diagnostic score from this assay was better associated with tumor malignancy and burden.CONCLUSIONUrine tumor DNA methylation assessment for early diagnosis, minimal, residual tumor detection and surveillance in BCa is a rapid, high-throughput, noninvasive, and promising approach, which may reduce the burden of cystoscopy and blind second surgery.FUNDINGThis study was supported by the National Key Research and Development Program of China and the National Natural Science Foundation of China.
Keywords: Cancer; Genetics; Molecular diagnosis; Oncology; Urology.
Conflict of interest statement
Figures




Comment in
-
Uro-Science.J Urol. 2021 Aug;206(2):480-482. doi: 10.1097/JU.0000000000001849. Epub 2021 May 12. J Urol. 2021. PMID: 33975458 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

