Antigen-specific B cell depletion for precision therapy of mucosal pemphigus vulgaris
- PMID: 32817591
- PMCID: PMC7685721
- DOI: 10.1172/JCI138416
Antigen-specific B cell depletion for precision therapy of mucosal pemphigus vulgaris
Abstract
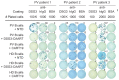
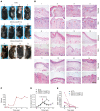
Desmoglein 3 chimeric autoantibody receptor T cells (DSG3-CAART) expressing the pemphigus vulgaris (PV) autoantigen DSG3 fused to CD137-CD3ζ signaling domains, represent a precision cellular immunotherapy approach for antigen-specific B cell depletion. Here, we present definitive preclinical studies enabling a first-in-human trial of DSG3-CAART for mucosal PV. DSG3-CAART specifically lysed human anti-DSG3 B cells from PV patients and demonstrated activity consistent with a threshold dose in vivo, resulting in decreased target cell burden, decreased serum and tissue-bound autoantibodies, and increased DSG3-CAART engraftment. In a PV active immune model with physiologic anti-DSG3 IgG levels, DSG3-CAART inhibited antibody responses against pathogenic DSG3 epitopes and autoantibody binding to epithelial tissues, leading to clinical and histologic resolution of blisters. DSG3 autoantibodies stimulated DSG3-CAART IFN-γ secretion and homotypic clustering, consistent with an activated phenotype. Toxicology screens using primary human cells and high-throughput membrane proteome arrays did not identify off-target cytotoxic interactions. These preclinical data guided the trial design for DSG3-CAART and may help inform CAART preclinical development for other antibody-mediated diseases.
Keywords: Autoimmune diseases; Autoimmunity; B cells; Immunotherapy; Therapeutics.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

