Tumor vascular status controls oxygen delivery facilitated by infused polymerized hemoglobins with varying oxygen affinity
- PMID: 32817659
- PMCID: PMC7462268
- DOI: 10.1371/journal.pcbi.1008157
Tumor vascular status controls oxygen delivery facilitated by infused polymerized hemoglobins with varying oxygen affinity
Abstract
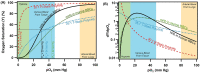
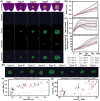
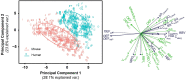
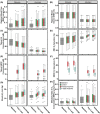
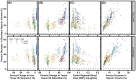
Oxygen (O2) delivery facilitated by hemoglobin (Hb)-based O2 carriers (HBOCs) is a promising strategy to increase the effectiveness of chemotherapeutics for treatment of solid tumors. However, the heterogeneous vascular structures present within tumors complicates evaluating the oxygenation potential of HBOCs within the tumor microenvironment. To account for spatial variations in the vasculature and tumor tissue that occur during tumor growth, we used a computational model to develop artificial tumor constructs. With these simulated tumors, we performed a polymerized human hemoglobin (hHb) (PolyhHb) enhanced oxygenation simulation accounting for differences in the physiologic characteristics of human and mouse blood. The results from this model were used to determine the potential effectiveness of different treatment options including a top load (low volume) and exchange (large volume) infusion of a tense quaternary state (T-State) PolyhHb, relaxed quaternary state (R-State) PolyhHb, and a non O2 carrying control. Principal component analysis (PCA) revealed correlations between the different regimes of effectiveness within the different simulated dosage options. In general, we found that infusion of T-State PolyhHb is more likely to decrease tissue hypoxia and modulate the metabolic rate of O2 consumption. Though the developed models are not a definitive descriptor of O2 carrier interaction in tumor capillary networks, we accounted for factors such as non-uniform vascular density and permeability that limit the applicability of O2 carriers during infusion. Finally, we have used these validated computational models to establish potential benchmarks to guide tumor treatment during translation of PolyhHb mediated therapies into clinical applications.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Robinson MF, Dupuis NP, Kusumoto T, Liu F, Menon K, Teicher Ba. Increased Tumor Oxygenation and Radiation Sensitivity in two Rat Tumors by A Hemoglobin-Based, Oxygen-Carrying Preparation. Artificial Cells, Blood Substitutes and Biotechnology. 1995;23(3):431–438. 10.3109/10731199509117959 - DOI - PubMed

