Tocilizumab and steroid treatment in patients with COVID-19 pneumonia
- PMID: 32817707
- PMCID: PMC7440633
- DOI: 10.1371/journal.pone.0237831
Tocilizumab and steroid treatment in patients with COVID-19 pneumonia
Abstract
Introduction: Coronavirus disease 2019 (COVID-19) can lead to respiratory failure due to severe immune response. Treatment targeting this immune response might be beneficial but there is limited evidence on its efficacy. The aim of this study was to determine if early treatment of patients with COVID-19 pneumonia with tocilizumab and/or steroids was associated with better outcome.
Methods: This observational single-center study included patients with COVID-19 pneumonia who were not intubated and received either standard of care (SOC, controls) or SOC plus early (within 3 days from hospital admission) anti-inflammatory treatment. SOC consisted of hydroxychloroquine 400mg bid plus, in those admitted before March 24th, also darunavir/ritonavir. Anti-inflammatory treatment consisted of either tocilizumab (8mg/kg intravenously or 162mg subcutaneously) or methylprednisolone 1 mg/kg for 5 days or both. Failure was defined as intubation or death, and the endpoints were failure-free survival (primary endpoint) and overall survival (secondary) at day 30. Difference between the groups was estimated as Hazard Ratio by a propensity score weighted Cox regression analysis (HROW).
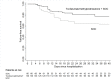
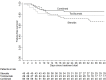
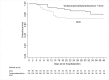
Results: Overall, 196 adults were included in the analyses. They were mainly male (67.4%), with comorbidities (78.1%) and severe COVID-19 pneumonia (83.7%). Median age was 67.9 years (range, 30-100) and median PaO2/FiO2 200 mmHg (IQR 133-289). Among them, 130 received early anti-inflammatory treatment with: tocilizumab (n = 29, 22.3%), methylprednisolone (n = 45, 34.6%), or both (n = 56, 43.1%). The adjusted failure-free survival among tocilizumab/methylprednisolone/SOC treated patients vs. SOC was 80.8% (95%CI, 72.8-86.7) vs. 64.1% (95%CI, 51.3-74.0), HROW 0.48, 95%CI, 0.23-0.99; p = 0.049. The overall survival among tocilizumab/methylprednisolone/SOC patients vs. SOC was 85.9% (95%CI, 80.7-92.6) vs. 71.9% (95%CI, 46-73), HROW 0.41, 95%CI: 0.19-0.89, p = 0.025.
Conclusion: Early adjunctive treatment with tocilizumab, methylprednisolone or both may improve outcomes in non-intubated patients with COVID-19 pneumonia.
Conflict of interest statement
The authors have declared that no competing interests exist
Figures

References
-
- Severe acute respiratory infections treatment centre: practical manual to set up and manage a SARI treatment centre and SARI screening facility in health care facilities. Geneva: World Health Organization; 2020. (WHO/2019-nCoV/SARI_treatment_center/2020.1.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

