This is a preprint.
Establishment of a Pediatric COVID-19 Biorepository: Unique Considerations and Opportunities for Studying the Impact of the COVID-19 Pandemic on Children
- PMID: 32818214
- PMCID: PMC7430592
- DOI: 10.21203/rs.3.rs-42030/v2
Establishment of a Pediatric COVID-19 Biorepository: Unique Considerations and Opportunities for Studying the Impact of the COVID-19 Pandemic on Children
Update in
-
Establishment of a pediatric COVID-19 biorepository: unique considerations and opportunities for studying the impact of the COVID-19 pandemic on children.BMC Med Res Methodol. 2020 Sep 11;20(1):228. doi: 10.1186/s12874-020-01110-y. BMC Med Res Methodol. 2020. PMID: 32917141 Free PMC article.
Abstract
Background: COVID-19, the disease caused by the highly infectious and transmissible coronavirus SARS-CoV-2, has quickly become a morbid global pandemic. Although the impact of SARS-CoV-2 infection in children is less clinically apparent, collecting high-quality biospecimens from infants, children, and adolescents in a standardized manner during the COVID-19 pandemic is essential to establish a biologic understanding of the disease in the pediatric population. This biorepository enables pediatric centers world-wide to collect samples uniformly to drive forward our understanding of COVID-19 by addressing specific pediatric and neonatal COVID-19-related questions.
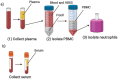
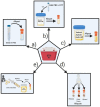
Methods: A COVID-19 biospecimen collection study was implemented with strategic enrollment guidelines to include patients seen in urgent care clinics and hospital settings, neonates born to SARS-CoV-2 infected mothers, and asymptomatic children. The methodology described here, details the importance of establishing collaborations between the clinical and research teams to harmonize protocols for patient recruitment and sample collection, processing and storage. It also details modifications required for biobanking during a surge of the COVID-19 pandemic.
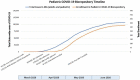
Results: Considerations and challenges facing enrollment of neonatal and pediatric cohorts are described. A roadmap is laid out for successful collection, processing, storage and database management of multiple pediatric samples such as blood, nasopharyngeal and oropharyngeal swabs, sputum, saliva, tracheal aspirates, stool, and urine. Using this methodology, we enrolled 327 participants, who provided a total of 972 biospecimens.
Conclusions: Pediatric biospecimens will be key in answering questions relating to viral transmission by children, differences between pediatric and adult viral susceptibility and immune responses, the impact of maternal SARS-CoV-2 infection on fetal development, and factors driving the Multisystem Inflammatory Syndrome in Children. The specimens in this biorepository will allow necessary comparative studies between children and adults, help determine the accuracy of current pediatric viral testing techniques, in addition to, understanding neonatal exposure to SARS-CoV-2 infection and disease abnormalities. The successful establishment of a pediatric biorepository is critical to provide insight into disease pathogenesis, and subsequently, develop future treatment and vaccination strategies.
Keywords: Biobank; Biorepository; COVID-19; Multisystem Inflammatory Syndrome in Children (MIS-C); Pediatric; SARS-CoV-2; Viral susceptibility; Viral transmission.
Conflict of interest statement
Competing Interests: The authors declare no competing interest.
Figures

Similar articles
-
Establishment of a pediatric COVID-19 biorepository: unique considerations and opportunities for studying the impact of the COVID-19 pandemic on children.BMC Med Res Methodol. 2020 Sep 11;20(1):228. doi: 10.1186/s12874-020-01110-y. BMC Med Res Methodol. 2020. PMID: 32917141 Free PMC article.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Boyum A: Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol 1976, Suppl 5:9–15. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

