The International Collaboration for Cancer Classification and Research
- PMID: 32818326
- PMCID: PMC7756795
- DOI: 10.1002/ijc.33260
The International Collaboration for Cancer Classification and Research
Abstract
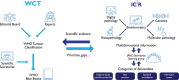
Gaps in the translation of research findings to clinical management have been recognized for decades. They exist for the diagnosis as well as the management of cancer. The international standards for cancer diagnosis are contained within the World Health Organization (WHO) Classification of Tumours, published by the International Agency for Research on Cancer (IARC) and known worldwide as the WHO Blue Books. In addition to their relevance to individual patients, these volumes provide a valuable contribution to cancer research and surveillance, fulfilling an important role in scientific evidence synthesis and international standard setting. However, the multidimensional nature of cancer classification, the way in which the WHO Classification of Tumours is constructed, and the scientific information overload in the field pose important challenges for the translation of research findings to tumour classification and hence cancer diagnosis. To help address these challenges, we have established the International Collaboration for Cancer Classification and Research (IC3 R) to provide a forum for the coordination of efforts in evidence generation, standard setting and best practice recommendations in the field of tumour classification. The first IC3 R meeting, held in Lyon, France, in February 2019, gathered representatives of major institutions involved in tumour classification and related fields to identify and discuss translational challenges in data comparability, standard setting, quality management, evidence evaluation and copyright, as well as to develop a collaborative plan for addressing these challenges.
Keywords: International Agency for Research on Cancer (IARC); WHO Tumour Classification; cancer research; evidence-based pathology; international standards.
© 2020 International Agency for Research on Cancer (IARC/WHO); licensed by UICC.
Conflict of interest statement
Dr Nicola Normanno reports grants and/or personal fees from Amgen, MSD, Quiagen, Roche, BMS, Merck, Thermofisher, Astrazeneca, Zanofi, ELI Lilly, Archer Dx and Illumina outside the submitted work and being President of the International Quality Network for Pathology (IQN Path) and the Italian Cancer Society (SIC). Dr Richard L. Schilsky reports his institution (ASCO) receives funding on his behalf from Astra‐Zeneca, Bayer, Boehringer‐Ingelheim, Bristol Myers Squibb, Eli Lilly & Co., Genentech, Merck, Pfizer. Dr Stefan Holdenrieder reports grants from Roche Diagnostics and Volition RX, as well as being a Scientific Advisory Board Member and consultant of Volition RX. The other authors declare that they have no conflict of interest. The other authors indicated no financial relationships.
Figures


References
-
- Edelman ER, FitzGerald GA. A decade of science translational medicine. Sci Transl Med. 2019;11(489):eaax4327. - PubMed
-
- Sartor RB. Translational research: bridging the widening gap between basic and clinical research. Gastroenterology. 2003;124(5):1178. - PubMed
-
- (EMBL‐EBI) TEBI . Experimental Factor Ontology The European Molecular Biology Laboratory (EMBL); 2020. https://www.ebi.ac.uk/
-
- Brazma A, Hingamp P, Quackenbush J, et al. Minimum information about a microarray experiment (MIAME)—toward standards for microarray data. Nat Genet. 2001;29(4):365‐371. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

