Breast cancer mammospheres secrete Adrenomedullin to induce lipolysis and browning of adjacent adipocytes
- PMID: 32819314
- PMCID: PMC7441622
- DOI: 10.1186/s12885-020-07273-7
Breast cancer mammospheres secrete Adrenomedullin to induce lipolysis and browning of adjacent adipocytes
Abstract
Background: Cancer cells cooperate with cells that compose their environment to promote tumor growth and invasion. Among them, adipocytes provide lipids used as a source of energy by cancer cells and adipokines that contribute to tumor expansion. Mechanisms supporting the dynamic interactions between cancer cells and stromal adipocytes, however, remain unclear.
Methods: We set-up a co-culture model with breast cancer cells grown in 3D as mammospheres and human adipocytes to accurately recapitulate intrinsic features of tumors, such as hypoxia and cancer cell-adipocytes interactions.
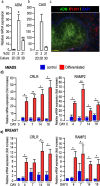
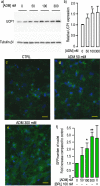
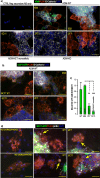
Results: Herein, we observed that the lipid droplets' size was reduced in adipocytes adjacent to the mammospheres, mimicking adipocyte morphology on histological sections. We showed that the uncoupling protein UCP1 was expressed in adipocytes close to tumor cells on breast cancer histological sections as well as in adipocytes in contact with the mammospheres. Mammospheres produced adrenomedullin (ADM), a multifactorial hypoxia-inducible peptide while ADM receptors were detected in adipocytes. Stimulation of adipocytes with ADM promoted UCP1 expression and increased HSL phosphorylation, which activated lipolysis. Invalidation of ADM in breast cancer cells dramatically reduced UCP1 expression in adipocytes.
Conclusions: Breast tumor cells secreted ADM that modified cancer-associated adipocytes through paracrine signaling, leading to metabolic changes and delipidation. Hence, ADM appears to be crucial in controlling the interactions between cancer cells and adipocytes and represents an excellent target to hinder them.
Keywords: Adipocytes; Adrenomedullin; Breast cancer; Browning; Lipolysis; UCP1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Peritumoral adipose tissue promotes lipolysis and white adipocytes browning by paracrine action.Front Endocrinol (Lausanne). 2023 Apr 26;14:1144016. doi: 10.3389/fendo.2023.1144016. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37181035 Free PMC article.
-
Utilization of adipocyte-derived lipids and enhanced intracellular trafficking of fatty acids contribute to breast cancer progression.Cell Commun Signal. 2018 Jun 18;16(1):32. doi: 10.1186/s12964-018-0221-6. Cell Commun Signal. 2018. PMID: 29914512 Free PMC article.
-
Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue.Endocrinology. 2005 Jun;146(6):2699-708. doi: 10.1210/en.2004-1424. Epub 2005 Mar 10. Endocrinology. 2005. PMID: 15761041
-
Drilling for Oil: Tumor-Surrounding Adipocytes Fueling Cancer.Trends Cancer. 2020 Jul;6(7):593-604. doi: 10.1016/j.trecan.2020.03.001. Epub 2020 Mar 26. Trends Cancer. 2020. PMID: 32610069 Review.
-
Molecular insights into the interplay between adiposity, breast cancer and bone metastasis.Clin Exp Metastasis. 2021 Apr;38(2):119-138. doi: 10.1007/s10585-021-10076-0. Epub 2021 Feb 16. Clin Exp Metastasis. 2021. PMID: 33591548 Review.
Cited by
-
Cancer-Associated Adipocytes in Breast Cancer: Causes and Consequences.Int J Mol Sci. 2021 Apr 6;22(7):3775. doi: 10.3390/ijms22073775. Int J Mol Sci. 2021. PMID: 33917351 Free PMC article. Review.
-
The evolving view of thermogenic fat and its implications in cancer and metabolic diseases.Signal Transduct Target Ther. 2022 Sep 16;7(1):324. doi: 10.1038/s41392-022-01178-6. Signal Transduct Target Ther. 2022. PMID: 36114195 Free PMC article. Review.
-
Associations of Plasma Bioactive Adrenomedullin Levels with Cardiovascular Risk Factors in BRCA1/2 Mutation Carriers.Geburtshilfe Frauenheilkd. 2022 Jun 3;82(6):601-609. doi: 10.1055/a-1811-2164. eCollection 2022 Jun. Geburtshilfe Frauenheilkd. 2022. PMID: 35903716 Free PMC article.
-
Adrenomedullin in Tumorigenesis and Cancer Progression.Int J Mol Sci. 2025 Jun 10;26(12):5552. doi: 10.3390/ijms26125552. Int J Mol Sci. 2025. PMID: 40565016 Free PMC article. Review.
-
Distinct Shades of Adipocytes Control the Metabolic Roles of Adipose Tissues: From Their Origins to Their Relevance for Medical Applications.Biomedicines. 2021 Jan 5;9(1):40. doi: 10.3390/biomedicines9010040. Biomedicines. 2021. PMID: 33466493 Free PMC article. Review.
References
-
- Tan J, Buache E, Chenard MP, Dali-Youcef N, Rio MC. Adipocyte is a non-trivial, dynamic partner of breast cancer cells. Int J Dev Biol. 2011;55(7–9):851–859. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

