Insulin: too much of a good thing is bad
- PMID: 32819363
- PMCID: PMC7441661
- DOI: 10.1186/s12916-020-01688-6
Insulin: too much of a good thing is bad
Abstract
Background: Insulin shares a limited physiological concentration range with other endocrine hormones. Not only too low, but also too high systemic insulin levels are detrimental for body functions.
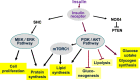
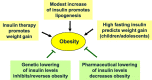
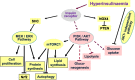
Main body: The physiological function and clinical relevance of insulin are usually seen in association with its role in maintaining glucose homeostasis. However, insulin is an anabolic hormone which stimulates a large number of cellular responses. Not only too low, but also excess insulin concentrations are detrimental to the physiological balance. Although the glucoregulatory activity of insulin is mitigated during hyperinsulinemia by dampening the efficiency of insulin signaling ("insulin resistance"), this is not the case for most other hormonal actions of insulin, including the promotion of protein synthesis, de novo lipogenesis, and cell proliferation; the inhibition of lipolysis, of autophagy-dependent cellular turnover, and of nuclear factor E2-related factor-2 (Nrf2)-dependent antioxidative; and other defense mechanisms. Hence, there is no general insulin resistance but selective impairment of insulin signaling which causes less glucose uptake from the blood and reduced activation of endothelial NO synthase (eNOS). Because of the largely unrestricted insulin signaling, hyperinsulinemia increases the risk of obesity, type 2 diabetes, and cardiovascular disease and decreases health span and life expectancy. In epidemiological studies, high-dose insulin therapy is associated with an increased risk of cardiovascular disease. Randomized controlled trials of insulin treatment did not observe any effect on disease risk, but these trials only studied low insulin doses up to 40 IU/day. Proof for a causal link between elevated insulin levels and cardiovascular disease risk comes from Mendelian randomization studies comparing individuals with genetically controlled low or high insulin production.
Conclusions: The detrimental actions of prolonged high insulin concentrations, seen also in cell culture, argue in favor of a lifestyle that limits circadian insulin levels. The health risks associated with hyperinsulinemia may have implications for treatment regimens used in type 2 diabetes.
Keywords: Autophagy; Cardiovascular morbidity and mortality; Hyperinsulinemia; Inflammation; Insulin resistance; Nrf2; Obesity; Oxidative stress; Type 2 diabetes mellitus; eNOS.
Conflict of interest statement
S. Martin has received non-restricted support for the conduct of trials of lifestyle change in persons at risk or with type 2 diabetes by Novartis Pharma GmbH, Boehringer Ingelheim Pharma GmbH & Co. KG, Almased Wellness GmbH, Nintendo of Europe GmbH, HMM Holding AG, and Gesellschaft von Freunden und Förderern der Heinrich-Heine-Universität Düsseldorf e.V.. H. Kolb, K. Kempf, and M. Röhling have nothing to disclose.
Figures


References
-
- Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, Salvatore P, et al. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996;12:106–109. - PubMed
-
- Unal EB, Uhlitz F, Bluthgen N. A compendium of ERK targets. FEBS Lett. 2017;591:2607–2615. - PubMed
-
- Williams KJ, Wu X. Imbalanced insulin action in chronic over nutrition: clinical harm, molecular mechanisms, and a way forward. Atherosclerosis. 2016;247:225–282. - PubMed
-
- Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75:155–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

