The CARDIA-trial protocol: a multinational, prospective, randomized, clinical trial comparing transthoracic esophagectomy with transhiatal extended gastrectomy in adenocarcinoma of the gastroesophageal junction (GEJ) type II
- PMID: 32819399
- PMCID: PMC7439687
- DOI: 10.1186/s12885-020-07152-1
The CARDIA-trial protocol: a multinational, prospective, randomized, clinical trial comparing transthoracic esophagectomy with transhiatal extended gastrectomy in adenocarcinoma of the gastroesophageal junction (GEJ) type II
Abstract
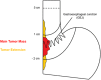
Background: Adenocarcinoma of the gastroesophageal junction (GEJ) Siewert type II can be resected by transthoracic esophagectomy or transhiatal extended gastrectomy. Both allow for a complete tumor resection, yet there is an ongoing controversy about which surgical approach is superior with regards to quality of life, oncological outcomes and survival. While some studies suggest a better oncological outcome after transthoracic esophagectomy, others favor transhiatal extended gastrectomy for a better postoperative quality of life. To date, only retrospective studies are available, showing ambiguous results.
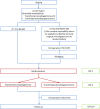
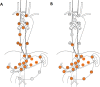
Methods: This study is a multinational, multicenter, randomized, clinical superiority trial. Patients (n = 262) with a GEJ type II tumor resectable by both transthoracic esophagectomy and transhiatal extended gastrectomy will be enrolled in the trial. Type II tumors are defined as tumors with their midpoint between ≤1 cm proximal and ≤ 2 cm distal of the top of gastric folds on preoperative endoscopy. Patients will be included in one of the participating European sites and are randomized to either transthoracic esophagectomy or transhiatal extended gastrectomy. The trial is powered to show superiority for esophagectomy with regards to the primary efficacy endpoint overall survival. Key secondary endpoints are complete resection (R0), number and localization of tumor infiltrated lymph nodes at dissection, post-operative complications, disease-free survival, quality of life and cost-effectiveness. Postoperative survival and quality of life will be followed-up for 24 months after discharge. Further survival follow-up will be conducted as quarterly phone calls up to 60 months.
Discussion: To date, as level 1 evidence is lacking, there is no consensus on which surgery is superior and both surgeries are used to treat GEJ type II carcinoma worldwide. The CARDIA trial is the first randomized trial to compare transthoracic esophagectomy versus transhiatal extended gastrectomy in patients with GEJ type II tumors. Several quality control measures were implemented in the protocol to ensure data reliability and increase the trial's significance. It is hypothesized that esophagectomy allows for a higher rate of radical resections and a more complete mediastinal lymph node dissection, resulting in a longer overall survival, while still providing an acceptable quality of life and cost-effectiveness.
Trial registration: The trial was registered on August 2nd 2019 at the German Clinical Trials Register under the trial-ID DRKS00016923 .
Keywords: Siewert type II; cardia carcinoma; esophageal adenocarcinoma; esophagectomy; gastrectomy; gastroesophageal junction.
Conflict of interest statement
LAAB is an Associate Editor for BMC Cancer. All other authors declare that they have no competing interests.
Figures
References
-
- Nederlandse Kankerregistratie. Cijfers over kanker. 2015. http://www.cijfersoverkanker.nl/. Accessed June, 2016.
-
- Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85(11):1457–1459. - PubMed
-
- McColl KE, Going JJ. Aetiology and classification of adenocarcinoma of the gastro-oesophageal junction/cardia. Gut. 2010;59(3):282–284. - PubMed
-
- Cancer Genome Atlas Research Network; Analysis Working Group: Asan University; BC Cancer Agency; Brigham and Women’s Hospital; Broad Institute; Brown University; Case Western Reserve University; Dana-Farber Cancer Institute; Duke University; Greater Poland Cancer Centre; Harvard Medical School; Institute for Systems Biology; KU Leuven; Mayo Clinic; Memorial Sloan Kettering Cancer Center; National Cancer Institute; Nationwide Children’s Hospital; Stanford University; University of Alabama; University of Michigan; University of North Carolina; University of Pittsburgh; University of Rochester; University of Southern California; University of Texas MD Anderson Cancer Center; University of Washington; Van Andel Research Institute; Vanderbilt University; Washington University; Genome Sequencing Center: Broad Institute; Washington University in St. Louis; Genome Characterization Centers: BC Cancer Agency; Broad Institute; Harvard Medical School; Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University; University of North Carolina; University of Southern California Epigenome Center; University of Texas MD Anderson Cancer Center; Van Andel Research Institute; Genome Data Analysis Centers: Broad Institute; Brown University; Harvard Medical School; Institute for Systems Biology; Memorial Sloan Kettering Cancer Center; University of California Santa Cruz; University of Texas MD Anderson Cancer Center; Biospecimen Core Resource: International Genomics Consortium; Research Institute at Nationwide Children’s Hospital; Tissue Source Sites: Analytic Biologic Services; Asan Medical Center; Asterand Bioscience; Barretos Cancer Hospital; BioreclamationIVT; Botkin Municipal Clinic; Chonnam National University Medical School; Christiana Care Health System; Cureline; Duke University; Emory University; Erasmus University; Indiana University School of Medicine; Institute of Oncology of Moldova; International Genomics Consortium; Invidumed; Israelitisches Krankenhaus Hamburg; Keimyung University School of Medicine; Memorial Sloan Kettering Cancer Center; National Cancer Center Goyang; Ontario Tumour Bank; Peter MacCallum Cancer Centre; Pusan National University Medical School; Ribeirão Preto Medical School; St. Joseph’s Hospital &Medical Center; St. Petersburg Academic University; Tayside Tissue Bank; University of Dundee; University of Kansas Medical Center; University of Michigan; University of North Carolina at Chapel Hill; University of Pittsburgh School of Medicine; University of Texas MD Anderson Cancer Center; Disease Working Group: Duke University; Memorial Sloan Kettering Cancer Center; National Cancer Institute; University of Texas MD Anderson Cancer Center; Yonsei University College of Medicine; Data Coordination Center: CSRA Inc.; Project Team: National Institutes of Health. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541(7636):169–175. - PMC - PubMed
-
- Botterweck AA, Schouten LJ, Volovics A, Dorant E, van Den Brandt PA. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol. 2000;29(4):645–654. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

