The gut microbiota regulates autism-like behavior by mediating vitamin B6 homeostasis in EphB6-deficient mice
- PMID: 32819434
- PMCID: PMC7441571
- DOI: 10.1186/s40168-020-00884-z
The gut microbiota regulates autism-like behavior by mediating vitamin B6 homeostasis in EphB6-deficient mice
Abstract
Background: Autism spectrum disorder (ASD) is a developmental disorder, and the effective pharmacological treatments for the core autistic symptoms are currently limited. Increasing evidence, particularly that from clinical studies on ASD patients, suggests a functional link between the gut microbiota and the development of ASD. However, the mechanisms linking the gut microbiota with brain dysfunctions (gut-brain axis) in ASD have not yet been full elucidated. Due to its genetic mutations and downregulated expression in patients with ASD, EPHB6, which also plays important roles in gut homeostasis, is generally considered a candidate gene for ASD. Nonetheless, the role and mechanism of EPHB6 in regulating the gut microbiota and the development of ASD are unclear.
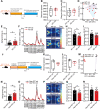
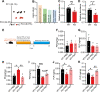
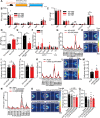
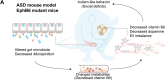
Results: Here, we found that the deletion of EphB6 induced autism-like behavior and disturbed the gut microbiota in mice. More importantly, transplantation of the fecal microbiota from EphB6-deficient mice resulted in autism-like behavior in antibiotic-treated C57BL/6J mice, and transplantation of the fecal microbiota from wild-type mice ameliorated the autism-like behavior in EphB6-deficient mice. At the metabolic level, the disturbed gut microbiota in EphB6-deficient mice led to vitamin B6 and dopamine defects. At the cellular level, the excitation/inhibition (E/I) balance in the medial prefrontal cortex was regulated by gut microbiota-mediated vitamin B6 in EphB6-deficient mice.
Conclusions: Our study uncovers a key role for the gut microbiota in the regulation of autism-like social behavior by vitamin B6, dopamine, and the E/I balance in EphB6-deficient mice, and these findings suggest new strategies for understanding and treating ASD. Video abstract.
Keywords: ASD; Dopamine; E/I balance; EphB6; Gut microbiota; Vitamin B6.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures





References
-
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383:896–910. - PubMed
-
- Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. - PubMed
-
- Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54:987–991. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

