Daratumumab, bortezomib, and dexamethasone in relapsed or refractory multiple myeloma: subgroup analysis of CASTOR based on cytogenetic risk
- PMID: 32819447
- PMCID: PMC7439722
- DOI: 10.1186/s13045-020-00948-5
Daratumumab, bortezomib, and dexamethasone in relapsed or refractory multiple myeloma: subgroup analysis of CASTOR based on cytogenetic risk
Abstract
Background: Multiple myeloma (MM) patients with high cytogenetic risk have poor outcomes. In CASTOR, daratumumab plus bortezomib/dexamethasone (D-Vd) prolonged progression-free survival (PFS) versus bortezomib/dexamethasone (Vd) alone and exhibited tolerability in patients with relapsed or refractory MM (RRMM).
Methods: This subgroup analysis evaluated D-Vd versus Vd in CASTOR based on cytogenetic risk, determined using fluorescence in situ hybridization and/or karyotype testing performed locally. High-risk patients had t(4;14), t(14;16), and/or del17p abnormalities. Minimal residual disease (MRD; 10-5 sensitivity threshold) was assessed via the clonoSEQ® assay V2.0. Of the 498 patients randomized, 40 (16%) in the D-Vd group and 35 (14%) in the Vd group were categorized as high risk.
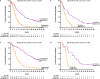
Results: After a median follow-up of 40.0 months, D-Vd prolonged median PFS versus Vd in patients with standard (16.6 vs 6.6 months; HR, 0.26; 95% CI, 0.19-0.37; P < 0.0001) and high (12.6 vs 6.2 months; HR, 0.41; 95% CI, 0.21-0.83; P = 0.0106) cytogenetic risk. D-Vd achieved deep responses, including higher rates of MRD negativity and sustained MRD negativity versus Vd, regardless of cytogenetic risk. The safety profile was consistent with the overall population of CASTOR.
Conclusion: These updated data reinforce the effectiveness and tolerability of daratumumab-based regimens for RRMM, regardless of cytogenetic risk status.
Trial registration: ClinicalTrials.gov, NCT02136134 . Registered 12 May 2014.
Keywords: Clinical trials; Multiple myeloma; Myeloma therapy.
Conflict of interest statement
KW received honoraria from GlaxoSmithKline, Sanofi, Adaptive, Amgen, Bristol-Myers Squibb, Celgene, Janssen, and Takeda; served in a consulting or advisory role for GlaxoSmithKline, Amgen, Adaptive, Bristol-Myers Squibb, Celgene, Janssen, Takeda, Sanofi, and Juno; and received institutional research funding from Amgen, Celgene, Sanofi, and Janssen. AS received honoraria from Celgene, Janssen, Amgen, AbbVie, Servier, and Takeda; served in a consulting or advisory role for Celgene, Janssen, Servier, and AbbVie; and served on a speakers bureau for and received institutional funding from Janssen, Takeda, and Celgene. SL holds stock and patents, royalties, or other intellectual property from Caelum Biosciences; served in a consulting or advisory role for Caelum Biosciences, Takeda, Janssen, Celgene, Bristol-Myers Squibb, AbbVie, and Bayer; received research funding from Sanofi; declared another relationship with Clinical Care Options; and served on data safety monitoring boards for Sorrento and Bayer. TMM holds stock in AbbVie; received honoraria and research funding from Celgene; and served in a consulting or advisory role for Janssen, Takeda, and Adaptive. M-DL is a consultant for and receives honoraria and travel support from AbbVie, Celgene, Amgen, and Janssen. AB received honoraria from Amgen; served in a consulting or advisory role for Takeda; and had travel, accommodations, or other expenses paid or reimbursed by Celgene, Janssen, and Amgen. VH served in a consulting or advisory role and on a speakers bureau for AbbVie, Amgen, Celgene, Janssen, Takeda, and Bristol-Myers Squibb. MCavo received honoraria from Celgene, Janssen, Amgen, Bristol-Myers Squibb, AbbVie, and Takeda; served in a consulting or advisory role for Janssen, Celgene, Amgen, and AbbVie; and served on a speakers bureau for and had travel, accommodations, or other expenses paid or reimbursed by Janssen and Celgene. AN received honoraria from and served in a consulting or advisory role for Janssen, GlaxoSmithKline, Celgene, Amgen, Takeda, Spectrum, Bristol-Myers Squibb, Karyopharm, Oncopeptides, and Adaptive and received research funding from Janssen, GlaxoSmithKline, Celgene, Amgen, Takeda, Karyopharm, and Bristol-Myers Squibb. HQ served in a consulting or advisory role for Celgene, Amgen, Karyopharm, and GlaxoSmithKline and received research funding from Celgene and Amgen. MM received honoraria from Janssen, Bristol-Myers Squibb, Celgene, and Amgen; served in a consulting or advisory role for Janssen, Bristol-Myers Squibb, Takeda, Celgene, Amgen, and Heidelberg Pharma; received research funding from Incyte and Bristol-Myers Squibb; and had travel, accommodations, or other expenses paid or reimbursed by Janssen, Bristol-Myers Squibb, Takeda, Celgene, and Amgen. CL received honoraria from and consulted for Amgen, Janssen-Cilag, Takeda, and Celgene. PC received honoraria from Daiichi Sankyo, Kite, KiowaKirin, Celgene, Janssen, Novartis, Roche, Takeda, Sanofi, Amgen, Gilead, and AbbVie and had travel, accommodations, or other expenses paid or reimbursed by Novartis, Janssen, Celgene, Bristol-Myers Squibb, Takeda, Gilead, Amgen, and AbbVie. AAC-K received research funding from the Mayo Clinic. NH served on the board of directors or advisory committees for Janssen. MCapra received honoraria from AbbVie, GlaxoSmithKline, Bristol-Myers Squibb, Adaptive, Takeda, Janssen, and Celgene. MB served in a consulting or advisory role and on a speakers bureau for Amgen, Janssen, and Takeda. RO consulted for Janssen. PS received honoraria from and research funding from Amgen, Celgene, Janssen, SkylineDx, and Takeda. TC is an employee of Janssen and holds stock options from Johnson & Johnson. ND, HA, JU, and RK are employees of Janssen. M-VM received honoraria from and served in a consulting or advisory role for Janssen, Celgene, Amgen, Takeda, GlaxoSmithKline, AbbVie, Seattle Genetics, and Adaptive. HA-L, IS, TM, BL, J-JL, WB, C-KM, J-CJ, and H-JS have no conflicts of interest to disclose.
Figures

References
-
- Lammerts van Bueren J, Jakobs D, Kaldenhoven N, Roza M, Hiddingh S, Meesters J, et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood. 2014;124(21):3474. doi: 10.1182/blood.V124.21.3474.3474. - DOI
-
- Overdijk MB, Verploegen S, Bogels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7(2):311–321. doi: 10.1080/19420862.2015.1007813. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

