Antinociceptive, reinforcing, and pruritic effects of the G-protein signalling-biased mu opioid receptor agonist PZM21 in non-human primates
- PMID: 32819621
- PMCID: PMC7565907
- DOI: 10.1016/j.bja.2020.06.057
Antinociceptive, reinforcing, and pruritic effects of the G-protein signalling-biased mu opioid receptor agonist PZM21 in non-human primates
Abstract
Background: A novel G-protein signalling-biased mu opioid peptide (MOP) receptor agonist, PZM21, was recently developed with a distinct chemical structure. It is a potent Gi/o activator with minimal β-arrestin-2 recruitment. Despite intriguing activity in rodent models, PZM21 function in non-human primates is unknown. The aim of this study was to investigate PZM21 actions after systemic or intrathecal administration in primates.
Methods: Antinociceptive, reinforcing, and pruritic effects of PZM21 were compared with those of the clinically used MOP receptor agonists oxycodone and morphine in assays of acute thermal nociception, capsaicin-induced thermal allodynia, itch scratching responses, and drug self-administration in gonadally intact, adult rhesus macaques (10 males, six females).
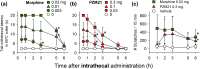
Results: After subcutaneous administration, PZM21 (1.0-6.0 mg kg-1) and oxycodone (0.1-0.6 mg kg-1) induced dose-dependent thermal antinociceptive effects (P<0.05); PZM21 was 10 times less potent than oxycodone. PZM21 exerted oxycodone-like reinforcing effects and strength as determined by two operant schedules of reinforcement in the intravenous drug self-administration assay. After intrathecal administration, PZM21 (0.03-0.3 mg) dose-dependently attenuated capsaicin-induced thermal allodynia (P<0.05). Although intrathecal PZM21 and morphine induced MOP receptor-mediated antiallodynic effects, both compounds induced robust, long-lasting itch scratching.
Conclusions: PZM21 induced antinociceptive, reinforcing, and pruritic effects similar to clinically used MOP receptor agonists in primates. Although structure-based discovery of PZM21 identified a novel avenue for studying G-protein signalling-biased ligands, biasing an agonist towards G-protein signalling pathways did not determine or alter reinforcing (i.e. abuse potential) or pruritic effects of MOP receptor agonists in a translationally relevant non-human primate model.
Keywords: G protein; opioid; opoid addiction; pruritus; rhesus macaque; spinal analgesics.
Copyright © 2020 British Journal of Anaesthesia. Published by Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Functional characterization of a novel opioid, PZM21, and its effects on the behavioural responses to morphine.Br J Pharmacol. 2019 Dec;176(23):4434-4445. doi: 10.1111/bph.14805. Epub 2019 Dec 8. Br J Pharmacol. 2019. PMID: 31347704 Free PMC article.
-
The G-protein-biased agents PZM21 and TRV130 are partial agonists of μ-opioid receptor-mediated signalling to ion channels.Br J Pharmacol. 2019 Sep;176(17):3110-3125. doi: 10.1111/bph.14702. Epub 2019 Jul 9. Br J Pharmacol. 2019. PMID: 31074038 Free PMC article.
-
The G-protein biased mu-opioid agonist, TRV130, produces reinforcing and antinociceptive effects that are comparable to oxycodone in rats.Drug Alcohol Depend. 2018 Nov 1;192:158-162. doi: 10.1016/j.drugalcdep.2018.08.002. Epub 2018 Sep 18. Drug Alcohol Depend. 2018. PMID: 30261403 Free PMC article.
-
Biased versus Partial Agonism in the Search for Safer Opioid Analgesics.Molecules. 2020 Aug 25;25(17):3870. doi: 10.3390/molecules25173870. Molecules. 2020. PMID: 32854452 Free PMC article. Review.
-
Hot topics in opioid pharmacology: mixed and biased opioids.Br J Anaesth. 2019 Jun;122(6):e136-e145. doi: 10.1016/j.bja.2019.03.006. Epub 2019 Apr 19. Br J Anaesth. 2019. PMID: 31010646 Review.
Cited by
-
Effects of NOP-Related Ligands in Nonhuman Primates.Handb Exp Pharmacol. 2019;254:323-343. doi: 10.1007/164_2019_211. Handb Exp Pharmacol. 2019. PMID: 30879202 Free PMC article.
-
Influence of G protein-biased agonists of μ-opioid receptor on addiction-related behaviors.Pharmacol Rep. 2021 Aug;73(4):1033-1051. doi: 10.1007/s43440-021-00251-1. Epub 2021 Apr 9. Pharmacol Rep. 2021. PMID: 33835467 Free PMC article. Review.
-
Biased Opioid Ligands: Revolution or Evolution?Front Pain Res (Lausanne). 2021 Sep 24;2:722820. doi: 10.3389/fpain.2021.722820. eCollection 2021. Front Pain Res (Lausanne). 2021. PMID: 35295469 Free PMC article. Review.
-
Potential therapeutic targets for the treatment of opioid abuse and pain.Adv Pharmacol. 2022;93:335-371. doi: 10.1016/bs.apha.2021.09.002. Epub 2021 Nov 9. Adv Pharmacol. 2022. PMID: 35341570 Free PMC article. Review.
-
Contribution of G-Protein α-Subunits to Analgesia, Hyperalgesia, and Hyperalgesic Priming Induced by Subanalgesic and Analgesic Doses of Fentanyl and Morphine.J Neurosci. 2022 Feb 16;42(7):1196-1210. doi: 10.1523/JNEUROSCI.1982-21.2021. Epub 2021 Dec 29. J Neurosci. 2022. PMID: 34965973 Free PMC article.
References
-
- Hewson D.W., Struys M., Hardman J.G. Opioids: refining the perioperative role of God's own medicine. Br J Anaesth. 2019;122:e93–e95. - PubMed
-
- Egan T.D. Are opioids indispensable for general anaesthesia? Br J Anaesth. 2019;122:e127–e135. - PubMed
-
- Volkow N.D., McLellan A.T. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253–1263. - PubMed
-
- Higgins C., Smith B.H., Matthews K. Evidence of opioid-induced hyperalgesia in clinical populations after chronic opioid exposure: a systematic review and meta-analysis. Br J Anaesth. 2019;122:e114–e126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

