Performance and Feasibility of Recalls Completed Using the Automated Self-Administered 24-Hour Dietary Assessment Tool in Relation to Other Self-Report Tools and Biomarkers in the Interactive Diet and Activity Tracking in AARP (IDATA) Study
- PMID: 32819883
- PMCID: PMC7606702
- DOI: 10.1016/j.jand.2020.06.015
Performance and Feasibility of Recalls Completed Using the Automated Self-Administered 24-Hour Dietary Assessment Tool in Relation to Other Self-Report Tools and Biomarkers in the Interactive Diet and Activity Tracking in AARP (IDATA) Study
Abstract
Background: Automated Self-Administered 24-Hour Dietary Assessment Tool (ASA24) is a self-administered web-based tool designed to collect detailed dietary data at low cost in observational studies.
Objective: The objectives of this study were to describe, overall and by demographic groups, the performance and feasibility of ASA24-2011 recalls and compare Healthy Eating Index-2015 (HEI-2015) total and component scores to 4-day food records (4DFRs) and food frequency questionnaires (FFQs).
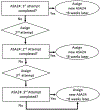
Design: Over 12 months, participants completed up to 6 ASA24 recalls, 2 web-based FFQs, and 2 unweighed paper-and-pencil 4DFRs. Up to 3 attempts were made to obtain each ASA24 recall. Participants were administered doubly-labeled water to provide a measure of total energy expenditure and collected two 24-hour urine samples to assess concentrations of nitrogen, sodium, and potassium.
Participants/setting: From January through September 2012, 1,110 adult members of AARP, 50 to 74 years of age, were recruited from the Pittsburgh, PA, area to participate in the Interactive Diet and Activity Tracking in AARP (IDATA) study. After excluding 33 participants who had not completed any dietary assessments, 531 men and 546 women remained.
Main outcome measures: Response rates, nutrient intakes compared to recovery biomarkers across each ASA24 administration day, and HEI-2015 total and component scores were measured.
Statistical analyses performed: Means, medians, standard deviations, interquartile ranges, and HEI-2015 total and component scores computed using a multivariate measurement error model are presented.
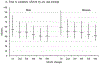
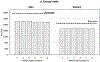
Results: Ninety-one percent of men and 86% of women completed 3 ASA24 recalls. Approximately three-quarters completed 5 or more, higher than the completion rates for 2 4DFRs and 2 FFQs. Approximately, three-quarters of men and 70% of women completed ASA24 on the first attempt; 1 in 5 completed it on the second. Completion rates varied slightly by age and body mass index. Median time to complete ASA24-2011 (current version: ASA24-2020) declined with subsequent recalls from 55 to 41 minutes in men and from 58 to 42 minutes in women and was lowest in those younger than 60 years. Mean nutrient intakes were similar across recalls. For each recording day, energy intakes estimated by ASA24 were lower than energy expenditure. Reported intakes for protein, potassium, and sodium were closer to recovery biomarkers for women, but not for men. Geometric means of reported intakes of these nutrients did not systematically vary across ASA24 administrations, but differences between reported intakes and biomarkers differed by nutrient. Of 100 possible points, HEI-2015 total scores were nearly identical for 4DFRs and ASA24 recalls and higher for FFQs (men: 61, 60, and 68; women: 64, 64, and 72, respectively).
Conclusions: ASA24, a freely available dietary assessment tool for use in large-scale nutrition research, was found to be highly feasible. Similar to previously reported data for nutrient intakes, HEI-2015 total and component scores for ASA24 recalls were comparable to those for 4DFRs, but not FFQs.
Trial registration: ClinicalTrials.gov identifier: NCT03268577 (http://www.clinicaltrials.gov).
Keywords: 4-day food record; ASA24; Feasibility; Food frequency questionnaire.
Copyright © 2020 Academy of Nutrition and Dietetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures






Similar articles
-
Comparison of self-reported dietary intakes from the Automated Self-Administered 24-h recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers.Am J Clin Nutr. 2018 Jan 1;107(1):80-93. doi: 10.1093/ajcn/nqx002. Am J Clin Nutr. 2018. PMID: 29381789 Free PMC article. Clinical Trial.
-
Comparing Reported Dietary Supplement Intakes between Two 24-Hour Recall Methods: The Automated Self-Administered 24-Hour Dietary Assessment Tool and the Interview-Administered Automated Multiple Pass Method.J Acad Nutr Diet. 2018 Jun;118(6):1080-1086. doi: 10.1016/j.jand.2018.02.013. J Acad Nutr Diet. 2018. PMID: 29803270 Free PMC article.
-
Administering a combination of online dietary assessment tools, the Automated Self-Administered 24-Hour Dietary Assessment Tool, and Diet History Questionnaire II, in a cohort of adults in Alberta's Tomorrow Project.J Acad Nutr Diet. 2021 Jul;121(7):1312-1326. doi: 10.1016/j.jand.2021.01.014. Epub 2021 Feb 18. J Acad Nutr Diet. 2021. PMID: 33612438
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Measurement Errors in Dietary Assessment Using Self-Reported 24-Hour Recalls in Low-Income Countries and Strategies for Their Prevention.Adv Nutr. 2017 Nov 15;8(6):980-991. doi: 10.3945/an.117.016980. Print 2017 Nov. Adv Nutr. 2017. PMID: 29141979 Free PMC article. Review.
Cited by
-
Potential of existing online 24-h dietary recall tools for national dietary surveys.Public Health Nutr. 2021 Nov;24(16):5361-5386. doi: 10.1017/S1368980021003517. Epub 2021 Aug 16. Public Health Nutr. 2021. PMID: 34392853 Free PMC article.
-
Development and validation of new predictive equations for the resting metabolic rate of older adults aged ≥65 y.Am J Clin Nutr. 2023 Jun;117(6):1164-1173. doi: 10.1016/j.ajcnut.2023.04.010. Epub 2023 Apr 11. Am J Clin Nutr. 2023. PMID: 37054885 Free PMC article.
-
Gut microbial features and dietary fiber intake predict gut microbiota response to resistant starch supplementation.Gut Microbes. 2024 Jan-Dec;16(1):2367301. doi: 10.1080/19490976.2024.2367301. Epub 2024 Jun 24. Gut Microbes. 2024. PMID: 38913541 Free PMC article.
-
Selecting a dietary assessment method for a national nutrition survey: a review and evaluation of online 24-h dietary recall tools.Public Health Nutr. 2024 Dec 5;27(1):e264. doi: 10.1017/S1368980024002507. Public Health Nutr. 2024. PMID: 39632717 Free PMC article. Review.
-
Dietary misreporting: a comparative study of recalls vs energy expenditure and energy intake by doubly-labeled water in older adults with overweight or obesity.BMC Med Res Methodol. 2025 Apr 26;25(1):115. doi: 10.1186/s12874-025-02568-4. BMC Med Res Methodol. 2025. PMID: 40287632 Free PMC article. Clinical Trial.
References
-
- Subar AF, Kushi LH, Lerman JL, Freedman LS. Invited Commentary: The Contribution to the Field of Nutritional Epidemiology of the Landmark 1985 Publication by Willett et al American journal of epidemiology. 2017;185(11):1124–1129. - PubMed
-
- Day N, McKeown N, Wong M, Welch A, Bingham S. Epidemiological assessment of diet: a comparison of a 7-day diary with a food frequency questionnaire using urinary markers of nitrogen, potassium and sodium. International journal of epidemiology. 2001;30(2):309–317. - PubMed
-
- Bingham SA, Gill C, Welch A, et al. Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. International journal of epidemiology. 1997;26 Suppl 1:S137–151. - PubMed
-
- Subar AF, Kipnis V, Troiano RP, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. American journal of epidemiology. 2003;158(1):1–13. - PubMed
-
- Schatzkin A, Kipnis V, Carroll RJ, et al. A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. International journal of epidemiology. 2003;32(6):1054–1062. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

