Cost-utility of sofosbuvir/velpatasvir versus other direct-acting antivirals for chronic hepatitis C genotype 1b infection in China
- PMID: 32819983
- PMCID: PMC7443302
- DOI: 10.1136/bmjopen-2019-035224
Cost-utility of sofosbuvir/velpatasvir versus other direct-acting antivirals for chronic hepatitis C genotype 1b infection in China
Abstract
Objective: This study aimed to estimate the cost-utility of sofosbuvir/velpatasvir (SOF/VEL) compared with other direct-acting antivirals (DAAs) in Chinese patients with hepatitis C virus (HCV).
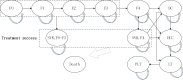
Design: A Markov model was developed to estimate the disease progression of patients with HCV over a lifetime horizon from the healthcare system perspective. Efficacy, clinical inputs and utilities were derived from the published literature. Drug costs were from the market price survey, and health costs for Markov health states were sourced from a Chinese study. Costs and utilities were discounted at an annual rate of 5%. One-way and probabilistic sensitivity analyses were conducted to test the impact of input parameters on the results.
Interventions: SOF/VEL was compared with sofosbuvir+ribavirin (SR), sofosbuvir+dasabuvir (SD), daclatasvir+asunaprevir (DCV/ASV), ombitasvir/paritaprevir/ritonavir+dasabuvir (3D) and elbasvir/grazoprevir (EBR/GZR).
Primary and secondary outcomes: Costs, quality-adjusted life years (QALYs) and incremental cost-utility ratios (ICURs).
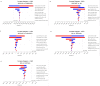
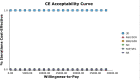
Results: SOF/VEL was economically dominant over SR and SD. However, 3D was economically dominant compared with SOF/VEL. Compared with DCV/ASV, SOF/VEL was cost-effective with the ICUR of US$1522 per QALY. Compared with EBR/GZR, it was not cost-effective with the ICUR of US$369 627 per QALY. One-way sensitivity analysis demonstrated that reducing the cost of SOF/VEL to the lower value of CI resulted in dominance over EBR/GZR and 3D. Probabilistic sensitivity analysis demonstrated that 3D was cost-effective in 100% of iterations in patients with genotype (GT) 1b and SOF/VEL was not cost-effective.
Conclusions: Compared with other oral DAA agents, SOF/VEL treatment was not the most cost-effectiveness option for patients with chronic HCV GT1b in China. Lower the price of SOF/VEL will make it cost-effective while simplifying treatment and achieving the goal of HCV elimination.
Keywords: health economics; hepatology; public health.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- World Health Organization Hepatitis C. fact sheet. Available: http://www.who.int/mediacentre/factsheets/fs164/en/ [Accessed 27 Jul 2019].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
