Succination inactivates gasdermin D and blocks pyroptosis
- PMID: 32820063
- PMCID: PMC8744141
- DOI: 10.1126/science.abb9818
Succination inactivates gasdermin D and blocks pyroptosis
Abstract
Activated macrophages undergo a metabolic switch to aerobic glycolysis, accumulating Krebs' cycle intermediates that alter transcription of immune response genes. We extended these observations by defining fumarate as an inhibitor of pyroptotic cell death. We found that dimethyl fumarate (DMF) delivered to cells or endogenous fumarate reacts with gasdermin D (GSDMD) at critical cysteine residues to form S-(2-succinyl)-cysteine. GSDMD succination prevents its interaction with caspases, limiting its processing, oligomerization, and capacity to induce cell death. In mice, the administration of DMF protects against lipopolysaccharide shock and alleviates familial Mediterranean fever and experimental autoimmune encephalitis by targeting GSDMD. Collectively, these findings identify GSDMD as a target of fumarate and reveal a mechanism of action for fumarate-based therapeutics that include DMF, for the treatment of multiple sclerosis.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

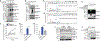
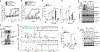

Comment in
-
Preventing pores and inflammation.Science. 2020 Sep 25;369(6511):1564-1565. doi: 10.1126/science.abe0917. Science. 2020. PMID: 32973018 No abstract available.
-
Metabolic regulation of pyroptotic cell death expands the therapeutic landscape for treating inflammatory disease.Signal Transduct Target Ther. 2021 Jan 29;6(1):37. doi: 10.1038/s41392-021-00467-w. Signal Transduct Target Ther. 2021. PMID: 33514689 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

