Inhibition of inflammatory CCR2 signaling promotes aged muscle regeneration and strength recovery after injury
- PMID: 32820177
- PMCID: PMC7441393
- DOI: 10.1038/s41467-020-17620-8
Inhibition of inflammatory CCR2 signaling promotes aged muscle regeneration and strength recovery after injury
Abstract
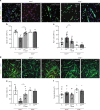
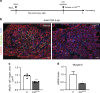
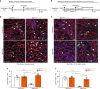
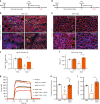
Muscle regeneration depends on a robust albeit transient inflammatory response. Persistent inflammation is a feature of age-related regenerative deficits, yet the underlying mechanisms are poorly understood. Here, we find inflammatory-related CC-chemokine-receptor 2 (Ccr2) expression in non-hematopoietic myogenic progenitors (MPs) during regeneration. After injury, the expression of Ccr2 in MPs corresponds to the levels of its ligands, the chemokines Ccl2, 7, and 8. We find stimulation of Ccr2-activity inhibits MP fusion and contribution to myofibers. This occurs in association with increases in MAPKp38δ/γ signaling, MyoD phosphorylation, and repression of the terminal myogenic commitment factor Myogenin. High levels of Ccr2-chemokines are a feature of regenerating aged muscle. Correspondingly, deletion of Ccr2 in MPs is necessary for proper fusion into regenerating aged muscle. Finally, opportune Ccr2 inhibition after injury enhances aged regeneration and functional recovery. These results demonstrate that inflammatory-induced activation of Ccr2 signaling in myogenic cells contributes to aged muscle regenerative decline.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356:1026–1030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

