Peripheral serum metabolomic profiles inform central cognitive impairment
- PMID: 32820198
- PMCID: PMC7441317
- DOI: 10.1038/s41598-020-70703-w
Peripheral serum metabolomic profiles inform central cognitive impairment
Abstract
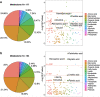
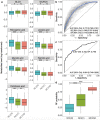
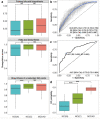
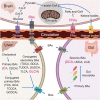
The incidence of Alzheimer's disease (AD) increases with age and is becoming a significant cause of worldwide morbidity and mortality. However, the metabolic perturbation behind the onset of AD remains unclear. In this study, we performed metabolite profiling in both brain (n = 109) and matching serum samples (n = 566) to identify differentially expressed metabolites and metabolic pathways associated with neuropathology and cognitive performance and to identify individuals at high risk of developing cognitive impairment. The abundances of 6 metabolites, glycolithocholate (GLCA), petroselinic acid, linoleic acid, myristic acid, palmitic acid, palmitoleic acid and the deoxycholate/cholate (DCA/CA) ratio, along with the dysregulation scores of 3 metabolic pathways, primary bile acid biosynthesis, fatty acid biosynthesis, and biosynthesis of unsaturated fatty acids showed significant differences across both brain and serum diagnostic groups (P-value < 0.05). Significant associations were observed between the levels of differential metabolites/pathways and cognitive performance, neurofibrillary tangles, and neuritic plaque burden. Metabolites abundances and personalized metabolic pathways scores were used to derive machine learning models, respectively, that could be used to differentiate cognitively impaired persons from those without cognitive impairment (median area under the receiver operating characteristic curve (AUC) = 0.772 for the metabolite level model; median AUC = 0.731 for the pathway level model). Utilizing these two models on the entire baseline control group, we identified those who experienced cognitive decline in the later years (AUC = 0.804, sensitivity = 0.722, specificity = 0.749 for the metabolite level model; AUC = 0.778, sensitivity = 0.633, specificity = 0.825 for the pathway level model) and demonstrated their pre-AD onset prediction potentials. Our study provides a proof-of-concept that it is possible to discriminate antecedent cognitive impairment in older adults before the onset of overt clinical symptoms using metabolomics. Our findings, if validated in future studies, could enable the earlier detection and intervention of cognitive impairment that may halt its progression.
Conflict of interest statement
R.K.D. is inventor on key patents in the field of metabolomics including applications for Alzheimer disease. M.A., R.B., and X.H. are co-inventors on patent WO2018049268 in this field. All other authors report no disclosures.
Figures

References
-
- Qiu C, De Ronchi D, Fratiglioni L. The epidemiology of the dementias: an update. Curr. Opin. Psychiatry. 2007;20:380–385. - PubMed
-
- Murphy, S. L., Xu, J., Kochanek, K. D. & Arias, E. Mortality in the United States, 2017. NCHS Data Brief (2018). - PubMed
-
- Graham WV, Bonito-Oliva A, Sakmar TP. Update on Alzheimer’s disease therapy and prevention strategies. Annu. Rev. Med. 2017;68:413–430. - PubMed
-
- Hulstaert F, et al. Improved discrimination of AD patients using -amyloid(1–42) and tau levels in CSF. Neurology. 1999;52:1555–1555. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

