RhCMV serostatus and vaccine adjuvant impact immunogenicity of RhCMV/SIV vaccines
- PMID: 32820216
- PMCID: PMC7441386
- DOI: 10.1038/s41598-020-71075-x
RhCMV serostatus and vaccine adjuvant impact immunogenicity of RhCMV/SIV vaccines
Abstract
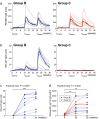
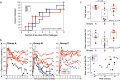
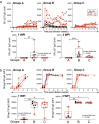
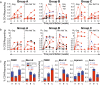
Rhesus cytomegalovirus (RhCMV) strain 68-1-vectored simian immunodeficiency virus (RhCMV/SIV) vaccines are associated with complete clearance of pathogenic SIV challenge virus, non-canonical major histocompatibility complex restriction, and absent antibody responses in recipients previously infected with wild-type RhCMV. This report presents the first investigation of RhCMV/SIV vaccines in RhCMV-seronegative macaques lacking anti-vector immunity. Fifty percent of rhesus macaques (RM) vaccinated with a combined RhCMV-Gag, -Env, and -Retanef (RTN) vaccine controlled pathogenic SIV challenge despite high peak viremia. However, kinetics of viral load control by vaccinated RM were considerably delayed compared to previous reports. Impact of a TLR5 agonist (flagellin; FliC) on vaccine efficacy and immunogenicity was also examined. An altered vaccine regimen containing an SIV Gag-FliC fusion antigen instead of Gag was significantly less immunogenic and resulted in reduced protection. Notably, RhCMV-Gag and RhCMV-Env vaccines elicited anti-Gag and anti-Env antibodies in RhCMV-seronegative RM, an unexpected contrast to vaccination of RhCMV-seropositive RM. These findings confirm that RhCMV-vectored SIV vaccines significantly protect against SIV pathogenesis. However, pre-existing vector immunity and a pro-inflammatory vaccine adjuvant may influence RhCMV/SIV vaccine immunogenicity and efficacy. Future investigation of the impact of pre-existing anti-vector immune responses on protective immunity conferred by this vaccine platform is warranted.
Conflict of interest statement
The University of California, W.L.W.C., D.J.H., P.A.B., and E.E.S. have a substantial financial interest in Tendel Therapies Inc., a company that may have a commercial interest in the results of this research. The potential individual and institutional conflicts of interest have been reviewed and managed by University of California Davis. Authors J.D.D., H.T.K., L.D.C., K.M., X.S., G.D.T., and B.L.S. declare no competing interests.
Figures


Similar articles
-
Utilizing a TLR5-Adjuvanted Cytomegalovirus as a Lentiviral Vaccine in the Nonhuman Primate Model for AIDS.PLoS One. 2016 May 16;11(5):e0155629. doi: 10.1371/journal.pone.0155629. eCollection 2016. PLoS One. 2016. PMID: 27182601 Free PMC article.
-
Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine.Nature. 2011 May 26;473(7348):523-7. doi: 10.1038/nature10003. Epub 2011 May 11. Nature. 2011. PMID: 21562493 Free PMC article.
-
Vaccination of Macaques with DNA Followed by Adenoviral Vectors Encoding Simian Immunodeficiency Virus (SIV) Gag Alone Delays Infection by Repeated Mucosal Challenge with SIV.J Virol. 2019 Oct 15;93(21):e00606-19. doi: 10.1128/JVI.00606-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31413132 Free PMC article.
-
Cytomegalovirus-vectored vaccines for HIV and other pathogens.AIDS. 2020 Mar 1;34(3):335-349. doi: 10.1097/QAD.0000000000002396. AIDS. 2020. PMID: 31634191 Free PMC article. Review.
-
Exploitation of Unconventional CD8 T-Cell Responses Induced by Engineered Cytomegaloviruses for the Development of an HIV-1 Vaccine.Vaccines (Basel). 2025 Jan 14;13(1):72. doi: 10.3390/vaccines13010072. Vaccines (Basel). 2025. PMID: 39852851 Free PMC article. Review.
Cited by
-
HIV T-cell immunogen design and delivery.Curr Opin HIV AIDS. 2022 Nov 1;17(6):333-337. doi: 10.1097/COH.0000000000000765. Epub 2022 Sep 19. Curr Opin HIV AIDS. 2022. PMID: 36165078 Free PMC article. Review.
-
Cytomegalovirus infection disrupts the influence of short-chain fatty acid producers on Treg/Th17 balance.Microbiome. 2022 Oct 10;10(1):168. doi: 10.1186/s40168-022-01355-3. Microbiome. 2022. PMID: 36210471 Free PMC article.
-
Recent Developments in NSG and NRG Humanized Mouse Models for Their Use in Viral and Immune Research.Viruses. 2023 Feb 9;15(2):478. doi: 10.3390/v15020478. Viruses. 2023. PMID: 36851692 Free PMC article. Review.

