Lyopreserved amniotic membrane is cellularly and clinically similar to cryopreserved construct for treating foot ulcers
- PMID: 32820605
- PMCID: PMC7754413
- DOI: 10.1111/iwj.13479
Lyopreserved amniotic membrane is cellularly and clinically similar to cryopreserved construct for treating foot ulcers
Abstract
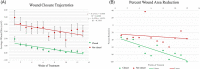
We compared cellular viability between cryopreserved and lyopreserved amniotic membranes and clinical outcomes of the lyopreserved construct in a prospective cohort study of 40 patients with neuropathic foot ulcers. Patients received weekly application of lyopreserved membrane for 12 weeks with standard weekly debridement and offloading. We evaluated the proportion of foot ulcers that closed, time to closure, closure trajectories, and infection during therapy. We used chi-square tests for dichotomous variables and independent t-tests for continuous variables with an alpha of α = .10. Cellular viability was equivalent between cryo- and lyopreserved amniotic tissues. Clinically, 48% of subjects' wounds closed in an average of 40.0 days. Those that did not close were older (63 vs 59 years, P = .011) and larger ulcers at baseline (7.8 vs 1.6 cm2 , P = .012). Significantly more patients who achieved closure reached a 50% wound area reduction in 4 weeks compared with non-closed wounds (73.7% vs 47.6%, P = .093). There was no difference in the slope of the wound closure trajectories between closed and non-closed wounds (0.124 and 0.159, P = .85), indicating the rate of closure was similar. The rate of closure was 0.60 mm/day (SD = 0.47) for wounds that closed and 0.50 mm/day (SD = 0.58) for wounds that did not close (P = .89).
Keywords: amniotic membrane; diabetic foot ulcer; infection; neuropathy.
© 2020 The Authors. International Wound Journal published by Medicalhelplines.com Inc (3M) and John Wiley & Sons Ltd.
Conflict of interest statement
Kathryn E. Davis has received research funding from EO2 Concepts, Inc.; Smith+Nephew; Integra; Cardinal Health, Astra Zeneca, Avazzia, and Pluristem.
Amanda L. Killeen has received research funding from EO2 Concepts, Inc.; Smith+Nephew; Integra; Cardinal Health, Astra Zeneca, Avazzia, and Pluristem.
David Farrar and Zachary D. Berriman‐Rozen declare no conflicts of interest.
Katherine M. Raspovic has received funding from Smith+Nephew and has consulted for Orthofix.
Matthew Malone has received funding from Medline Industries.
Lawrence A. Lavery has received research funding from EO2 Concepts, Inc.; Smith+Nephew; Integra; Cardinal Health, Astra Zeneca, Avazzia, and Pluristem.
Figures



References
-
- Johnson A, Gyurdieva A, Dhall S, Danilkovitch A, Duan‐Arnold Y. Understanding the impact of preservation methods on the integrity and functionality of placental allografts. Ann Plast Surg. 2017;79(2):203‐213. - PubMed
-
- von Versen‐Hoynck F, Syring C, Bachmann S, Moller DE. The influence of different preservation and sterilisation steps on the histological properties of amnion allografts—light and scanning electron microscopic studies. Cell Tissue Bank. 2004;5(1):45‐56. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

