Adherence to HIV antiretroviral therapy among pregnant and postpartum women during the Option B+ era: 12-month cohort study in urban South Africa and rural Uganda
- PMID: 32820622
- PMCID: PMC7441010
- DOI: 10.1002/jia2.25586
Adherence to HIV antiretroviral therapy among pregnant and postpartum women during the Option B+ era: 12-month cohort study in urban South Africa and rural Uganda
Abstract
Introduction: We conducted a cohort study to understand patterns of anti-retroviral therapy (ART) adherence during pregnancy, postpartum and non-pregnancy follow-up among women initiating ART in public clinics offering Option B+ in rural Uganda and urban South Africa.
Methods: We collected survey data, continuously monitored ART adherence (Wisepill), HIV-RNA and pregnancy tests at zero, six and twelve months from women initiating ART in Uganda and South Africa, 2015 to 2017. The primary predictor of interest was follow-up time categorized as pregnant (pregnancy diagnosis to pregnancy end), postpartum (pregnancy end to study exit) or non-pregnancy-related (neither pregnant nor postpartum). Fractional regression models included demographics and socio-behavioural factors informed by the Behavioral Model for Vulnerable Populations. We evaluated HIV-RNA at 12 months by ever- versus never-pregnant status.
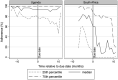
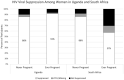
Results: In Uganda, 247 women contributed 676, 900 and 1274 months of pregnancy, postpartum and non-pregnancy-related follow-up. Median ART adherence was consistently ≥90%: pregnancy, 94% (interquartile range [IQR] 78,98); postpartum, 90% (IQR 70,97) and non-pregnancy, 90% (IQR 80,98). Poorer adherence was associated with younger age (0.98% [95% CI 0.33%, 1.62%] average increase per year of age) and higher CD4 cell count (1.01% [0.08%, 1.94%] average decrease per 50 cells/mm3 ). HIV-RNA was suppressed among 91% (N = 135) ever-pregnant and 86% (N = 85) never-pregnant women. In South Africa, 190 women contributed 259, 624 and 1247 months of pregnancy, postpartum and non-pregnancy-related follow-up. Median adherence was low during pregnancy, 74% (IQR 31,96); postpartum, 40% (IQR 4,65) and non-pregnancy, 77% (IQR 47,92). Poorer adherence was associated with postpartum status (22.3% [95%CI 8.6%, 35.4%] average decrease compared to non-pregnancy-related follow-up) and less emotional support (1.4% [0.22%, 2.58%] average increase per unit increase). HIV-RNA was suppressed among 57% (N = 47) ever-pregnant and 86% (N = 93) never-pregnant women.
Conclusions: Women in rural Uganda maintained high adherence with 91% of ever-pregnant and 86% of never-pregnant women suppressing HIV-RNA at 12 months. Women in urban South Africa struggled with adherence, particularly during postpartum follow-up with median adherence of 40% and 57% of women with HIV-RNA suppression at one year, suggesting a crisis for postpartum women with HIV in South Africa. Findings suggest that effective interventions should promote emotional support.
Keywords: ARV; Africa < Region; Cohort studies; HIV; adherence; cohort studies; gender; women.
© 2020 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of the International AIDS Society.
Figures
References
-
- World Health Organization . Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: summary of key features and recommendations, June 2013. 2013. - PubMed
-
- Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, Swai A, et al. Prevention of mother‐to‐child transmission of HIV‐1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52(3):406–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

