Modelling Toxoplasma gondii infection in human cerebral organoids
- PMID: 32820712
- PMCID: PMC7534270
- DOI: 10.1080/22221751.2020.1812435
Modelling Toxoplasma gondii infection in human cerebral organoids
Abstract
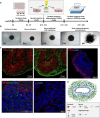
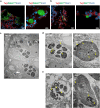
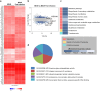
Pluripotent stem cell-derived cerebral organoids have the potential to recapitulate the pathophysiology of in vivo human brain tissue, constituting a valuable resource for modelling brain disorders, including infectious diseases. Toxoplasma gondii, an intracellular protozoan parasite, infects most warm-blooded animals, including humans, causing toxoplasmosis. In immunodeficient patients and pregnant women, infection often results in severe central nervous system disease and fetal miscarriage. However, understanding the molecular pathophysiology of the disease has been challenging due to limited in vitro model systems. Here, we developed a new in vitro model system of T. gondii infection using human brain organoids. We observed that tachyzoites can infect human cerebral organoids and are transformed to bradyzoites and replicate in parasitophorous vacuoles to form cysts, indicating that the T. gondii asexual life cycle is efficiently simulated in the brain organoids. Transcriptomic analysis of T. gondii-infected organoids revealed the activation of the type I interferon immune response against infection. In addition, in brain organoids, T. gondii exhibited a changed transcriptome related to protozoan invasion and replication. This study shows cerebral organoids as physiologically relevant in vitro model systems useful for advancing the understanding of T. gondii infections and host interactions.
Keywords: Cerebral organoid; Toxoplasma gondii; disease modelling; pluripotent stem cells; toxoplasmosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Similar articles
-
Use of in vitro derived human neuronal models to study host-parasite interactions of Toxoplasma gondii in neurons and neuropathogenesis of chronic toxoplasmosis.Front Cell Infect Microbiol. 2023 Mar 8;13:1129451. doi: 10.3389/fcimb.2023.1129451. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36968101 Free PMC article. Review.
-
From Entry to Early Dissemination-Toxoplasma gondii's Initial Encounter With Its Host.Front Cell Infect Microbiol. 2019 Mar 5;9:46. doi: 10.3389/fcimb.2019.00046. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 30891433 Free PMC article. Review.
-
Impact of Engineered Expression of Mitochondrial Association Factor 1b on Toxoplasma gondii Infection and the Host Response in a Mouse Model.mSphere. 2018 Oct 17;3(5):e00471-18. doi: 10.1128/mSphere.00471-18. mSphere. 2018. PMID: 30333181 Free PMC article.
-
Neuroprotective Effect of Chronic Intracranial Toxoplasma gondii Infection in a Mouse Cerebral Ischemia Model.Korean J Parasitol. 2020 Aug;58(4):461-466. doi: 10.3347/kjp.2020.58.4.461. Epub 2020 Aug 25. Korean J Parasitol. 2020. PMID: 32871641 Free PMC article.
-
[Toxoplasma gondii: a potential role in the genesis of psychiatric disorders].Encephale. 2013 Feb;39(1):38-43. doi: 10.1016/j.encep.2012.06.014. Epub 2012 Aug 21. Encephale. 2013. PMID: 23095600 Review. French.
Cited by
-
Leveraging iPSC technology to assess neuro-immune interactions in neurological and psychiatric disorders.Front Psychiatry. 2023 Nov 9;14:1291115. doi: 10.3389/fpsyt.2023.1291115. eCollection 2023. Front Psychiatry. 2023. PMID: 38025464 Free PMC article. Review.
-
Innate immune cell response to host-parasite interaction in a human intestinal tissue microphysiological system.Sci Adv. 2022 May 6;8(18):eabm8012. doi: 10.1126/sciadv.abm8012. Epub 2022 May 6. Sci Adv. 2022. PMID: 35544643 Free PMC article.
-
Transcending Dimensions in Apicomplexan Research: from Two-Dimensional to Three-Dimensional In Vitro Cultures.Microbiol Mol Biol Rev. 2022 Jun 15;86(2):e0002522. doi: 10.1128/mmbr.00025-22. Epub 2022 Apr 12. Microbiol Mol Biol Rev. 2022. PMID: 35412359 Free PMC article. Review.
-
Modeling SARS-CoV-2 infection in individuals with opioid use disorder with brain organoids.J Tissue Eng. 2021 Feb 26;12:2041731420985299. doi: 10.1177/2041731420985299. eCollection 2021 Jan-Dec. J Tissue Eng. 2021. PMID: 33738089 Free PMC article. Review.
-
Effects of diet and ovariectomy on Toxoplasma gondii brain infection: functional alterations and neuronal loss in rats.Brain Commun. 2024 Dec 31;7(1):fcae441. doi: 10.1093/braincomms/fcae441. eCollection 2025. Brain Commun. 2024. PMID: 39741781 Free PMC article.
References
-
- Da Gama LM, Ribeiro-Gomes FL, Guimaraes Jr U, et al. . Reduction in adhesiveness to extracellular matrix components, modulation of adhesion molecules and in vivo migration of murine macrophages infected with Toxoplasma gondii. Microbes Infect. 2004 Nov;6(14):1287–1296. doi: 10.1016/j.micinf.2004.07.008 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
