Metabolomic and Signaling Programs Induced by Immobilized versus Soluble IFN γ in Neural Stem Cells
- PMID: 32820900
- PMCID: PMC10286853
- DOI: 10.1021/acs.bioconjchem.0c00338
Metabolomic and Signaling Programs Induced by Immobilized versus Soluble IFN γ in Neural Stem Cells
Abstract
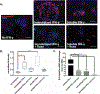
Neural stem cells (NSCs) provide a strategy to replace damaged neurons following traumatic central nervous system injuries. A major hurdle to translation of this therapy is that direct application of NSCs to CNS injury does not support sufficient neurogenesis due to lack of proper cues. To provide prolonged spatial cues to NSCs IFN-γ was immobilized to biomimetic hydrogel substrate to supply physical and biochemical signals to instruct the encapsulated NSCs to be neurogenic. However, the immobilization of factors, including IFN-γ, versus soluble delivery of the same factor, has been incompletely characterized especially with respect to activation of signaling and metabolism in cells over longer time points. In this study, protein and metabolite changes in NSCs induced by immobilized versus soluble IFN-γ at 7 days were evaluated. Soluble IFN-γ, refreshed daily over 7 days, elicited stronger responses in NSCs compared to immobilized IFN-γ, indicating that immobilization may not sustain signaling or has altered ligand/receptor interaction and integrity. However, both IFN-γ delivery types supported increased βIII tubulin expression in parallel with canonical and noncanonical receptor-signaling compared to no IFN-γ. Global metabolomics and pathway analysis revealed that soluble and immobilized IFN-γ altered metabolic pathway activities including energy, lipid, and amino acid synthesis, with soluble IFN-γ having the greatest metabolic impact overall. Finally, soluble and immobilized IFN-γ support mitochondrial voltage-dependent anion channel (VDAC) expression that correlates to differentiated NSCs. This work utilizes new methods to evaluate cell responses to protein delivery and provides insight into mode of action that can be harnessed to improve regenerative medicine-based strategies.
Figures
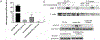



Similar articles
-
Covalent growth factor tethering to direct neural stem cell differentiation and self-organization.Acta Biomater. 2017 Apr 15;53:140-151. doi: 10.1016/j.actbio.2017.01.068. Epub 2017 Feb 2. Acta Biomater. 2017. PMID: 28161574 Free PMC article.
-
Concurrent Delivery of Soluble and Immobilized Proteins to Recruit and Differentiate Neural Stem Cells.Biomacromolecules. 2019 Sep 9;20(9):3445-3452. doi: 10.1021/acs.biomac.9b00719. Epub 2019 Aug 28. Biomacromolecules. 2019. PMID: 31460746 Free PMC article.
-
Differentiation of neural stem cells in three-dimensional growth factor-immobilized chitosan hydrogel scaffolds.Biomaterials. 2011 Jan;32(1):57-64. doi: 10.1016/j.biomaterials.2010.09.031. Epub 2010 Oct 8. Biomaterials. 2011. PMID: 20934216
-
Immunopharmacological intervention for successful neural stem cell therapy: New perspectives in CNS neurogenesis and repair.Pharmacol Ther. 2014 Jan;141(1):21-31. doi: 10.1016/j.pharmthera.2013.08.001. Epub 2013 Aug 15. Pharmacol Ther. 2014. PMID: 23954656 Review.
-
Methods of reactivation and reprogramming of neural stem cells for neural repair.Methods. 2018 Jan 15;133:3-20. doi: 10.1016/j.ymeth.2017.08.014. Epub 2017 Aug 31. Methods. 2018. PMID: 28864354 Review.
Cited by
-
Cellular and Molecular Insights into the Divergence of Neural Stem Cells on Matrigel and Poly-l-lysine Interfaces.ACS Appl Mater Interfaces. 2024 Jun 26;16(25):31922-31935. doi: 10.1021/acsami.4c02575. Epub 2024 Jun 14. ACS Appl Mater Interfaces. 2024. PMID: 38874539 Free PMC article.
-
Gold Nanostrip Array-Mediated Wireless Electrical Stimulation for Accelerating Functional Neuronal Differentiation.Adv Sci (Weinh). 2022 Aug;9(22):e2202376. doi: 10.1002/advs.202202376. Epub 2022 May 26. Adv Sci (Weinh). 2022. PMID: 35618610 Free PMC article.
-
Cell Biology Meets Cell Metabolism: Energy Production Is Similar in Stem Cells and in Cancer Stem Cells in Brain and Bone Marrow.J Histochem Cytochem. 2022 Jan;70(1):29-51. doi: 10.1369/00221554211054585. Epub 2021 Oct 29. J Histochem Cytochem. 2022. PMID: 34714696 Free PMC article. Review.
-
Radiotherapy Side Effects: Comprehensive Proteomic Study Unraveled Neural Stem Cell Degenerative Differentiation upon Ionizing Radiation.Biomolecules. 2022 Nov 26;12(12):1759. doi: 10.3390/biom12121759. Biomolecules. 2022. PMID: 36551187 Free PMC article.
-
A Review of the Evidence for Tryptophan and the Kynurenine Pathway as a Regulator of Stem Cell Niches in Health and Disease.Int J Tryptophan Res. 2024 May 15;17:11786469241248287. doi: 10.1177/11786469241248287. eCollection 2024. Int J Tryptophan Res. 2024. PMID: 38757094 Free PMC article. Review.
References
-
- Vieira MS, Santos AK, Vasconcellos R, Goulart VAM, Parreira RC, Kihara AH, Ulrich H, and Resende RR, Neural stem cell differentiation into mature neurons: Mechanisms of regulation and biotechnological applications. Biotechnol Adv, 2018. 36(7): p. 1946–1970. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

