Protein-Nanoparticle Interaction: Corona Formation and Conformational Changes in Proteins on Nanoparticles
- PMID: 32821101
- PMCID: PMC7418457
- DOI: 10.2147/IJN.S254808
Protein-Nanoparticle Interaction: Corona Formation and Conformational Changes in Proteins on Nanoparticles
Abstract
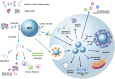
Nanoparticles (NPs) are highly potent tools for the diagnosis of diseases and specific delivery of therapeutic agents. Their development and application are scientifically and industrially important. The engineering of NPs and the modulation of their in vivo behavior have been extensively studied, and significant achievements have been made in the past decades. However, in vivo applications of NPs are often limited by several difficulties, including inflammatory responses and cellular toxicity, unexpected distribution and clearance from the body, and insufficient delivery to a specific target. These unfavorable phenomena may largely be related to the in vivo protein-NP interaction, termed "protein corona." The layer of adsorbed proteins on the surface of NPs affects the biological behavior of NPs and changes their functionality, occasionally resulting in loss-of-function or gain-of-function. The formation of a protein corona is an intricate process involving complex kinetics and dynamics between the two interacting entities. Structural changes in corona proteins have been reported in many cases after their adsorption on the surfaces of NPs that strongly influence the functions of NPs. Thus, understanding of the conformational changes and unfolding process of proteins is very important to accelerate the biomedical applications of NPs. Here, we describe several protein corona characteristics and specifically focus on the conformational fluctuations in corona proteins induced by NPs.
Keywords: conformational change; nanoparticle; protein corona; structure; surface characteristic.
© 2020 Park.
Conflict of interest statement
The author reports no conflicts of interest in this work.
Figures

Similar articles
-
Protein corona, understanding the nanoparticle-protein interactions and future perspectives: A critical review.Int J Biol Macromol. 2021 Feb 1;169:290-301. doi: 10.1016/j.ijbiomac.2020.12.108. Epub 2020 Dec 21. Int J Biol Macromol. 2021. PMID: 33340622 Review.
-
A health concern regarding the protein corona, aggregation and disaggregation.Biochim Biophys Acta Gen Subj. 2019 May;1863(5):971-991. doi: 10.1016/j.bbagen.2019.02.012. Epub 2019 Feb 22. Biochim Biophys Acta Gen Subj. 2019. PMID: 30802594 Free PMC article. Review.
-
In Situ Characterization of Protein Adsorption onto Nanoparticles by Fluorescence Correlation Spectroscopy.Acc Chem Res. 2017 Feb 21;50(2):387-395. doi: 10.1021/acs.accounts.6b00579. Epub 2017 Feb 1. Acc Chem Res. 2017. PMID: 28145686
-
The curious cases of nanoparticle induced amyloidosis during protein corona formation and anti-amyloidogenic nanomaterials: Paradox or prejudice?Int J Biol Macromol. 2021 Dec 15;193(Pt A):1009-1020. doi: 10.1016/j.ijbiomac.2021.10.195. Epub 2021 Oct 30. Int J Biol Macromol. 2021. PMID: 34728302 Review.
-
Understanding the nanoparticle-protein corona complexes using computational and experimental methods.Int J Biochem Cell Biol. 2016 Jun;75:162-74. doi: 10.1016/j.biocel.2016.02.008. Epub 2016 Feb 9. Int J Biochem Cell Biol. 2016. PMID: 26873405 Review.
Cited by
-
Sustainable polymeric adsorbents for adsorption-based water remediation and pathogen deactivation: a review.RSC Adv. 2024 Oct 21;14(45):33143-33190. doi: 10.1039/d4ra05269b. eCollection 2024 Oct 17. RSC Adv. 2024. PMID: 39434995 Free PMC article. Review.
-
Gold Nanoparticles Augment N-Terminal Cleavage and Splicing Reactions in Mycobacterium tuberculosis SufB.Front Bioeng Biotechnol. 2021 Dec 23;9:773303. doi: 10.3389/fbioe.2021.773303. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 35004641 Free PMC article.
-
Transthyretin: From Structural Stability to Osteoarticular and Cardiovascular Diseases.Cells. 2021 Jul 13;10(7):1768. doi: 10.3390/cells10071768. Cells. 2021. PMID: 34359938 Free PMC article. Review.
-
Identification of Proteins Using Supramolecular Gold Nanoparticle-Dye Sensor Arrays.Anal Sens. 2023 May;3(3):e202200080. doi: 10.1002/anse.202200080. Epub 2023 Jan 20. Anal Sens. 2023. PMID: 37250385 Free PMC article.
-
Protein corona formation on different-shaped CdSe/CdS semiconductor nanocrystals.Nanoscale Adv. 2024 Nov 25;7(2):560-571. doi: 10.1039/d4na00696h. eCollection 2025 Jan 14. Nanoscale Adv. 2024. PMID: 39650619 Free PMC article.
References
-
- Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed. 2014;53:12320–12364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

