Practice Advisory on the Appropriate Use of NSAIDs in Primary Care
- PMID: 32821151
- PMCID: PMC7422842
- DOI: 10.2147/JPR.S247781
Practice Advisory on the Appropriate Use of NSAIDs in Primary Care
Abstract
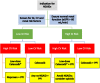
Cyclo-oxygenase (COX)-2 selective and nonselective nonsteroidal anti-inflammatory drugs (NSAIDs) are important in managing acute and chronic pain secondary to inflammation. As a greater understanding of the risks of gastrointestinal (GI), cardiovascular (CV) and renal events with NSAIDs use has emerged, guidelines have evolved to reflect differences in risks among NSAIDs. Updated guidelines have yet to reflect new evidence from recent trials which showed similar CV event rates with celecoxib compared to naproxen and ibuprofen, and significantly better GI tolerability for celecoxib. This practice advisory paper aims to present consensus statements and associated guidance regarding appropriate NSAID use based on a review of current evidence by a multidisciplinary group of expert clinicians. This paper is especially intended to guide primary care practitioners within Asia in the appropriate use of NSAIDs in primary care. Following a literature review, group members used a modified Delphi consensus process to determine agreement with selected recommendations. Agreement with a statement by 75% of total voting members was defined a priori as consensus. For low GI risk patients, any nonselective NSAID plus proton pump inhibitor (PPI) or celecoxib alone is acceptable treatment when CV risk is low; for high CV risk patients, low-dose celecoxib or naproxen plus PPI is appropriate. For high GI risk patients, celecoxib plus PPI is acceptable for low CV risk patients; low-dose celecoxib plus PPI is appropriate for high CV risk patients, with the alternative to avoid NSAIDs and consider opioids instead. Appropriate NSAID prescription assumes that the patient has normal renal function at commencement, with ongoing monitoring recommended. In conclusion, appropriate NSAID use requires consideration of all risks.
Keywords: COX-2 selective inhibitors; cardiovascular risk; gastrointestinal risk; inflammation; nonsteroidal anti-inflammatory drugs; osteoarthritis.
© 2020 Ho et al.
Conflict of interest statement
The meeting during which these recommendations were initially discussed was organized and funded by Pfizer. Pfizer representatives did not participate in discussions. KY Ho has received honoraria from Pfizer, Mundipharma, P&G, Avanos and A. Menarini, and reports non-financial support from MIMS HK (manuscript preparation) and personal fees from Pfizer for attending expert meeting during the conduct of the study, and personal fees from Menarini, Baxter Healthcare, Mundipharma, Johnson & Johnson, and Avanos, outside the submitted work. M Cardosa has received honoraria for giving lectures and participating in expert group discussions for Pfizer, Mundipharma, A. Menarini and GlaxoSmithKline and reports that IASP received Pfizer Independent Grants for Learning and Change (IGLC) grant in 2017 for the project “A Toolkit for Multidisciplinary Pain Clinics in Southeast Asia”. S Chaiamnuay has received honoraria from Pfizer, Amgen, Janssen-Cilag, Astellas and Novartis.HQT Ho has received honoraria from Pfizer, A. Menarini, Merck and Boehringer-Ingelheim. O Kamil has served on advisory boards for Amgen and Pfizer, and has received honoraria from Amgen, Pfizer, MIMS, Johnson & Johnson and Merck Sharp & Dohme. S Mokhtar has received honoraria from Pfizer, Lilly and Amgen. S Navarra has received honoraria from Pfizer, Abbott, Johnson & Johnson and Novartis. R Pinzon has received honoraria from Pfizer, P&G and Soho Pharma. S Tsuruoka has received honoraria from Pfizer, Astellas, AstraZeneca, Teijin Pharma and Tanabe-Mitsubishi Pharma. HB Yim served as a principal investigator for the CONDOR trial and has served on advisory boards and received honoraria from Takeda. E Choy has received research grants and/or personal fees from, and/or served as member of advisory boards and speaker bureaus of, AbbVie, Allergan, Amgen, AstraZeneca, Bio-Cancer, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Chugai Pharma, Daiichi Sankyo, Eli Lilly, Ferring Pharmaceutical, Gilead, GlaxoSmithKline, Hospira, ISIS, Jazz Pharmaceuticals, Janssen, MedImmune, Merrimack Pharmaceutical, Merck Sharp & Dohme, Napp, Novimmune, Novartis, ObsEva, Pfizer, Regeneron, Roche, R-Pharm, Sanofi, SynAct Pharma, Synovate, Tonix and UCB. All other authors report no conflicts of interest in this work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

