Fentanyl vapor self-administration model in mice to study opioid addiction
- PMID: 32821843
- PMCID: PMC7406365
- DOI: 10.1126/sciadv.abc0413
Fentanyl vapor self-administration model in mice to study opioid addiction
Abstract
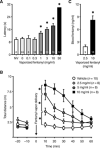
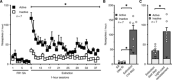
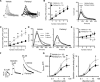
Intravenous drug self-administration is considered the "gold standard" model to investigate the neurobiology of drug addiction in rodents. However, its use in mice is limited by frequent complications of intravenous catheterization. Given the many advantages of using mice in biomedical research, we developed a noninvasive mouse model of opioid self-administration using vaporized fentanyl. Mice readily self-administered fentanyl vapor, titrated their drug intake, and exhibited addiction-like behaviors, including escalation of drug intake, somatic signs of withdrawal, drug intake despite punishment, and reinstatement of drug seeking. Electrophysiological recordings from ventral tegmental area dopamine neurons showed a lower amplitude of GABAB receptor-dependent currents during protracted abstinence from fentanyl vapor self-administration. This mouse model of fentanyl self-administration recapitulates key features of opioid addiction, overcomes limitations of the intravenous model, and allows investigation of the neurobiology of opioid addiction in unprecedented ways.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Kuczyńska K., Grzonkowski P., Kacprzak Ł., Zawilska J. B., Abuse of fentanyl: An emerging problem to face. Forensic Sci. Int. 289, 207–214 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

