Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial
- PMID: 32821939
- PMCID: PMC7442954
- DOI: 10.1001/jama.2020.16349
Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial
Abstract
Importance: Remdesivir demonstrated clinical benefit in a placebo-controlled trial in patients with severe coronavirus disease 2019 (COVID-19), but its effect in patients with moderate disease is unknown.
Objective: To determine the efficacy of 5 or 10 days of remdesivir treatment compared with standard care on clinical status on day 11 after initiation of treatment.
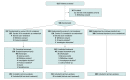
Design, setting, and participants: Randomized, open-label trial of hospitalized patients with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and moderate COVID-19 pneumonia (pulmonary infiltrates and room-air oxygen saturation >94%) enrolled from March 15 through April 18, 2020, at 105 hospitals in the United States, Europe, and Asia. The date of final follow-up was May 20, 2020.
Interventions: Patients were randomized in a 1:1:1 ratio to receive a 10-day course of remdesivir (n = 197), a 5-day course of remdesivir (n = 199), or standard care (n = 200). Remdesivir was dosed intravenously at 200 mg on day 1 followed by 100 mg/d.
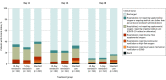
Main outcomes and measures: The primary end point was clinical status on day 11 on a 7-point ordinal scale ranging from death (category 1) to discharged (category 7). Differences between remdesivir treatment groups and standard care were calculated using proportional odds models and expressed as odds ratios. An odds ratio greater than 1 indicates difference in clinical status distribution toward category 7 for the remdesivir group vs the standard care group.
Results: Among 596 patients who were randomized, 584 began the study and received remdesivir or continued standard care (median age, 57 [interquartile range, 46-66] years; 227 [39%] women; 56% had cardiovascular disease, 42% hypertension, and 40% diabetes), and 533 (91%) completed the trial. Median length of treatment was 5 days for patients in the 5-day remdesivir group and 6 days for patients in the 10-day remdesivir group. On day 11, patients in the 5-day remdesivir group had statistically significantly higher odds of a better clinical status distribution than those receiving standard care (odds ratio, 1.65; 95% CI, 1.09-2.48; P = .02). The clinical status distribution on day 11 between the 10-day remdesivir and standard care groups was not significantly different (P = .18 by Wilcoxon rank sum test). By day 28, 9 patients had died: 2 (1%) in the 5-day remdesivir group, 3 (2%) in the 10-day remdesivir group, and 4 (2%) in the standard care group. Nausea (10% vs 3%), hypokalemia (6% vs 2%), and headache (5% vs 3%) were more frequent among remdesivir-treated patients compared with standard care.
Conclusions and relevance: Among patients with moderate COVID-19, those randomized to a 10-day course of remdesivir did not have a statistically significant difference in clinical status compared with standard care at 11 days after initiation of treatment. Patients randomized to a 5-day course of remdesivir had a statistically significant difference in clinical status compared with standard care, but the difference was of uncertain clinical importance.
Trial registration: ClinicalTrials.gov Identifier: NCT04292730.
Conflict of interest statement
Figures
Comment in
-
Efficacy of Remdesivir in COVID-19.JAMA. 2020 Sep 15;324(11):1041-1042. doi: 10.1001/jama.2020.16337. JAMA. 2020. PMID: 32821934 No abstract available.
-
Is remdesivir important in clinical practice as a treatment of COVID-19? A study based on meta-analysis data.Pol Arch Intern Med. 2021 Jan 29;131(1):96-97. doi: 10.20452/pamw.15686. Epub 2020 Nov 24. Pol Arch Intern Med. 2021. PMID: 33231938 No abstract available.
References
-
- World Health Organization Coronavirus Disease (COVID-19) Situation Report—202 Published August 9, 2020. Accessed August 10, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- Johns Hopkins Coronavirus Resource Center. Accessed August 10, 2020. https://coronavirus.jhu.edu/map.html
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

