MILO/ENGOT-ov11: Binimetinib Versus Physician's Choice Chemotherapy in Recurrent or Persistent Low-Grade Serous Carcinomas of the Ovary, Fallopian Tube, or Primary Peritoneum
- PMID: 32822286
- PMCID: PMC7655017
- DOI: 10.1200/JCO.20.01164
MILO/ENGOT-ov11: Binimetinib Versus Physician's Choice Chemotherapy in Recurrent or Persistent Low-Grade Serous Carcinomas of the Ovary, Fallopian Tube, or Primary Peritoneum
Abstract
Purpose: Low-grade serous ovarian carcinomas (LGSOCs) have historically low chemotherapy responses. Alterations affecting the MAPK pathway, most commonly KRAS/BRAF, are present in 30%-60% of LGSOCs. The purpose of this study was to evaluate binimetinib, a potent MEK1/2 inhibitor with demonstrated activity across multiple cancers, in LGSOC.
Methods: This was a 2:1 randomized study of binimetinib (45 mg twice daily) versus physician's choice chemotherapy (PCC). Eligible patients had recurrent measurable LGSOC after ≥ 1 prior platinum-based chemotherapy but ≤ 3 prior chemotherapy lines. The primary end point was progression-free survival (PFS) by blinded independent central review (BICR); additional assessments included overall survival (OS), overall response rate (ORR), duration of response (DOR), clinical-benefit rate, biomarkers, and safety.
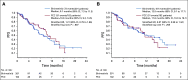
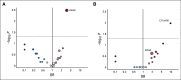
Results: A total of 303 patients were randomly assigned to an arm of the study at the time of interim analysis (January 20, 2016). Median PFS by BICR was 9.1 months (95% CI, 7.3 to 11.3) for binimetinib and 10.6 months (95% CI, 9.2 to 14.5) for PCC (hazard ratio,1.21; 95%CI, 0.79 to 1.86), resulting in early study closure according to a prespecified futility boundary after 341 patients had enrolled. Secondary efficacy end points were similar in the two groups: ORR 16% (complete response [CR]/partial responses[PRs], 32) versus 13% (CR/PRs, 13); median DOR, 8.1 months (range, 0.03 to ≥ 12.0 months) versus 6.7 months (0.03 to ≥ 9.7 months); and median OS, 25.3 versus 20.8 months for binimetinib and PCC, respectively. Safety results were consistent with the known safety profile of binimetinib; the most common grade ≥ 3 event was increased blood creatine kinase level (26%). Post hoc analysis suggests a possible association between KRAS mutation and response to binimetinib. Results from an updated analysis (n = 341; January 2019) were consistent.
Conclusion: Although the MEK Inhibitor in Low-Grade Serous Ovarian Cancer Study did not meet its primary end point, binimetinib showed activity in LGSOC across the efficacy end points evaluated. A higher response to chemotherapy than expected was observed and KRAS mutation might predict response to binimetinib.
Trial registration: ClinicalTrials.gov NCT01849874.
Figures

Comment in
-
MEK Inhibitors for the Treatment of Low-Grade Serous Ovarian Cancer: Expanding Therapeutic Options for a Rare Ovarian Cancer Subtype.J Clin Oncol. 2020 Nov 10;38(32):3731-3734. doi: 10.1200/JCO.20.02190. Epub 2020 Sep 8. J Clin Oncol. 2020. PMID: 32897828 No abstract available.
References
-
- Colombo N, Peiretti M, Parma G, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v23–v30. - PubMed
-
- Diaz-Padilla I, Malpica AL, Minig L, et al. Ovarian low-grade serous carcinoma: A comprehensive update. Gynecol Oncol. 2012;126:279–285. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

