Ipriflavone and Ipriflavone loaded albumin nanoparticles reverse lipopolysaccharide induced neuroinflammation in rats
- PMID: 32822403
- PMCID: PMC7446929
- DOI: 10.1371/journal.pone.0237929
Ipriflavone and Ipriflavone loaded albumin nanoparticles reverse lipopolysaccharide induced neuroinflammation in rats
Retraction in
-
Retraction: Ipriflavone and Ipriflavone loaded albumin nanoparticles reverse lipopolysaccharide induced neuroinflammation in rats.PLoS One. 2023 Nov 2;18(11):e0294102. doi: 10.1371/journal.pone.0294102. eCollection 2023. PLoS One. 2023. PMID: 37917647 Free PMC article. No abstract available.
Abstract
Background: Neuroinflammation causes neurodegenerative conditions like Alzheimer's disease (AD). Ipriflavone (IP), therapeutic compound to postmenopausal osteoporosis, has limited estrogenic activity and is accounted as AChE inhibitor. The developing of drug delivery systems to enable drug targeting to specific sites increases the drug therapeutic effect.
Objective: The aim of the present study was to formulate and evaluate ipriflavone loaded albumin nanoparticles (IP-Np) along with free ipriflavone against lipopolysaccharide (LPS) induced neuroinflammation in rats.
Methods: Neuroinflammation was induced by intra-peritoneal (i.p) injection of LPS (250 μg/kg rat body weight) then treatments were conducted with (1) ipriflavone at two doses 50 mg/kg and 5 mg/kg, (2) IP-Np (5 mg ipriflavone/kg) or (3) IP-Np coated with polysorbate 80 (IP-Np-T80) (5 mg ipriflavone/kg). The alteration of the inflammatory response in male adult Wistar rats' brain hippocampus was investigated by examining associated indices using biochemical and molecular analyses.
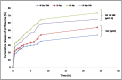
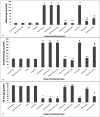
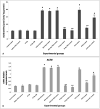
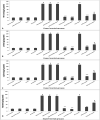
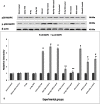
Results: A significant upsurge in inflammatory mediators and decline in antioxidant status were observed in LPS-induced rats. In one hand, ipriflavone (50 mg/kg), IP-Np and IP-Np-T80 ameliorated LPS induced brain hippocampal inflammation where they depreciated the level of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) and enhanced antioxidant status. In another hand, ipriflavone at dose (5 mg/kg) didn't show the same therapeutic effect.
Conclusion: The current study provides evidence for the potential neuroprotective effect of ipriflavone (50 mg/kg) against LPS-induced neuroinflammation in rats through its anti-inflammatory and antioxidant activities. Moreover, nanoparticles significantly attenuated neuroinflammation in concentration lower than the effective therapeutic dose of free drug ten times.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Neuroprotective effect of ipriflavone against scopolamine-induced memory impairment in rats.Psychopharmacology (Berl). 2017 Oct;234(20):3037-3053. doi: 10.1007/s00213-017-4690-x. Epub 2017 Jul 22. Psychopharmacology (Berl). 2017. PMID: 28733814
-
Berberine ameliorates lipopolysaccharide-induced learning and memory deficit in the rat: insights into underlying molecular mechanisms.Metab Brain Dis. 2019 Feb;34(1):245-255. doi: 10.1007/s11011-018-0349-5. Epub 2018 Nov 20. Metab Brain Dis. 2019. PMID: 30456649
-
Biochemical investigation and in silico analysis of the therapeutic efficacy of Ipriflavone through Tet-1 Surface-Modified-PLGA nanoparticles in Streptozotocin-Induced Alzheimer's like Disease: Reduced oxidative damage and etiological Descriptors.Int J Pharm. 2025 Jan 25;669:125021. doi: 10.1016/j.ijpharm.2024.125021. Epub 2024 Dec 2. Int J Pharm. 2025. PMID: 39631714
-
Evaluation of Insulin Resistance Induced Brain Tissue Dysfunction in Obese Dams and their Neonates: Role of Ipriflavone Amelioration.Comb Chem High Throughput Screen. 2021;24(6):767-780. doi: 10.2174/1386207323666200808181148. Comb Chem High Throughput Screen. 2021. PMID: 32772909
-
Soybean isoflavone ameliorates cognitive impairment, neuroinflammation, and amyloid β accumulation in a rat model of Alzheimer's disease.Environ Sci Pollut Res Int. 2019 Sep;26(25):26060-26070. doi: 10.1007/s11356-019-05862-z. Epub 2019 Jul 5. Environ Sci Pollut Res Int. 2019. PMID: 31278647
Cited by
-
Increasing the Power of Polyphenols through Nanoencapsulation for Adjuvant Therapy against Cardiovascular Diseases.Molecules. 2021 Jul 30;26(15):4621. doi: 10.3390/molecules26154621. Molecules. 2021. PMID: 34361774 Free PMC article. Review.
-
Ipriflavone ameliorates intervertebral disc degeneration by inhibiting osteoporosis of vertebral body and pyroptosis of the nucleus pulposus in instability of lumbar spine and diabetic mice.Front Bioeng Biotechnol. 2025 Aug 5;13:1639117. doi: 10.3389/fbioe.2025.1639117. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40837020 Free PMC article.
-
Effect of baicalin and baicalin-bovine serum albumin nanoparticle against bendiocarb exposure in rats.Toxicol Res (Camb). 2024 Sep 3;13(5):tfae134. doi: 10.1093/toxres/tfae134. eCollection 2024 Oct. Toxicol Res (Camb). 2024. PMID: 39233847 Free PMC article.
-
Comprehensive analyses identify potential biomarkers for encephalitis in HIV infection.Sci Rep. 2023 Oct 27;13(1):18418. doi: 10.1038/s41598-023-45922-6. Sci Rep. 2023. PMID: 37891420 Free PMC article.
-
Extraction, Radical Scavenging Activities, and Chemical Composition Identification of Flavonoids from Sunflower (Helianthus annuus L.) Receptacles.Molecules. 2021 Jan 14;26(2):403. doi: 10.3390/molecules26020403. Molecules. 2021. PMID: 33466694 Free PMC article.
References
-
- Meyer KC. Immunity, inflammation, and aging Inflammation, Advancing Age and Nutrition: Elsevier; 2014. p. 29–38.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

