Ixazomib, dexamethasone, and rituximab in treatment-naive patients with Waldenström macroglobulinemia: long-term follow-up
- PMID: 32822482
- PMCID: PMC7448596
- DOI: 10.1182/bloodadvances.2020001963
Ixazomib, dexamethasone, and rituximab in treatment-naive patients with Waldenström macroglobulinemia: long-term follow-up
Abstract
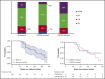
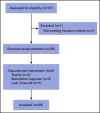
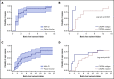
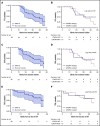
Proteasome inhibition is a standard of care for the primary treatment of patients with Waldenström macroglobulinemia (WM). We present the long-term follow-up of a prospective, phase II clinical trial that evaluated the combination of ixazomib, dexamethasone, and rituximab (IDR) in 26 treatment-naive patients with WM. IDR was administered as 6 monthly induction cycles followed by 6 every-2-month maintenance cycles. The MYD88 L265P mutation was detected in all patients, and CXCR4 mutations were detected in 15 patients (58%). The median time to response (TTR) and time to major response (TTMR) were 2 and 6 months, respectively. Patients with and without CXCR4 mutations had median TTR of 3 months and 1 month, respectively (P = .003), and median TTMR of 10 months and 3 months, respectively (P = .31). The overall, major, and very good partial response (VGPR) rates were 96%, 77%, and 19%, respectively. The rate of VGPR in patients with and without CXCR4 mutations were 7% and 36%, respectively (P = .06). The median progression-free survival (PFS) was 40 months, the median duration of response (DOR) was 38 months, and the median time to next treatment (TTNT) was 40 months. PFS, DOR, and TTNT were not affected by CXCR4 mutational status. The safety profile was excellent with no grade 4 adverse events or deaths to date. IDR provides a safe and effective frontline treatment option for symptomatic patients with WM. This study was registered at www.clinicaltrials.gov as #NCT02400437.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: J.J.C. has received honoraria and/or research funds from AbbVie, Beigene, Janssen, Kymera, Millennium, Pharmacyclics, and TG Therapeutics. S.P.T. has received research funding and/or consulting fees from BMS, Pharmacyclics, and Janssen. The remaining authors declare no competing financial interests.
Figures

References
-
- Dimopoulos MA, García-Sanz R, Gavriatopoulou M, et al. . Primary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): long-term results of a phase 2 study of the European Myeloma Network (EMN). Blood. 2013;122(19):3276-3282. - PubMed
-
- Ghobrial IM, Xie W, Padmanabhan S, et al. . Phase II trial of weekly bortezomib in combination with rituximab in untreated patients with Waldenström macroglobulinemia. Am J Hematol. 2010;85(9):670-674. - PubMed
-
- Treon SP, Tripsas CK, Meid K, et al. . Carfilzomib, rituximab, and dexamethasone (CaRD) treatment offers a neuropathy-sparing approach for treating Waldenström’s macroglobulinemia. Blood. 2014;124(4):503-510. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

