A batch-effect adjusted Simon's two-stage design for cancer vaccine clinical studies
- PMID: 32822525
- PMCID: PMC8713137
- DOI: 10.1111/biom.13358
A batch-effect adjusted Simon's two-stage design for cancer vaccine clinical studies
Abstract
In the development of cancer treatment vaccines, phase II clinical studies are conducted to examine the efficacy of a vaccine in order to screen out vaccines with minimal activity. Immune responses are commonly used as the primary endpoint for assessing vaccine efficacy. With respect to study design, Simon's two-stage design is a popular format for phase II cancer clinical studies because of its simplicity and ethical considerations. Nonetheless, it is not straightforward to apply Simon's two-stage design to cancer vaccine studies when performing immune assays in batches, as outcomes from multiple patients may be correlated with each other in the presence of batch effects. This violates the independence assumption of Simon's two-stage design. In this paper, we numerically explore the impact of batch effects on Simon's two-stage design, propose a batch-effect adjusted Simon's two-stage design, demonstrate the proposed design by both a simulation study and a therapeutic human papillomavirus vaccine trial, and briefly introduce a software that implements the proposed design.
Keywords: Simon's two-stage design; batch effect; cancer vaccine; clinical trial design; phase II study.
© 2020 The International Biometric Society.
Figures
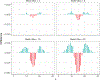
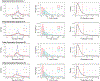

References
-
- Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, et al., (1996) Phenotypic analysis of antigen-specific t lymphocytes. Science, 274, 94–96. - PubMed
-
- Andtbacka R, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al., (2015) Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. Journal of Clinical Oncology, 33, 2780–2788. - PubMed
-
- Benito M, Parker J, Du Q, Wu J, Xiang D, Perou CM, et al., (2004) Adjustment of systematic microarray data biases. Bioinformatics, 20, 105–114. - PubMed
-
- BIPM, IEC, IFCC, ILAC, ISO, IUPAC, IUPAP and OIML. (2012) The international vocabulary of metrology–basic and general concepts and associated terms (vim), 3rd edition. jcgm 200: 2012. JCGM (Joint Committee for Guides in Metrology).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

