Genome-Scale Imaging of the 3D Organization and Transcriptional Activity of Chromatin
- PMID: 32822575
- PMCID: PMC7851072
- DOI: 10.1016/j.cell.2020.07.032
Genome-Scale Imaging of the 3D Organization and Transcriptional Activity of Chromatin
Abstract
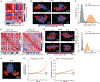
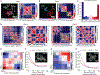
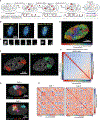
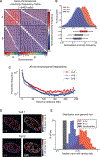
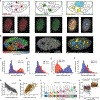
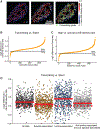
The 3D organization of chromatin regulates many genome functions. Our understanding of 3D genome organization requires tools to directly visualize chromatin conformation in its native context. Here we report an imaging technology for visualizing chromatin organization across multiple scales in single cells with high genomic throughput. First we demonstrate multiplexed imaging of hundreds of genomic loci by sequential hybridization, which allows high-resolution conformation tracing of whole chromosomes. Next we report a multiplexed error-robust fluorescence in situ hybridization (MERFISH)-based method for genome-scale chromatin tracing and demonstrate simultaneous imaging of more than 1,000 genomic loci and nascent transcripts of more than 1,000 genes together with landmark nuclear structures. Using this technology, we characterize chromatin domains, compartments, and trans-chromosomal interactions and their relationship to transcription in single cells. We envision broad application of this high-throughput, multi-scale, and multi-modal imaging technology, which provides an integrated view of chromatin organization in its native structural and functional context.
Keywords: 3D genome organization; MERFISH; chromosome compartments; chromosome conformation; genome-scale imaging; multiplexed FISH; nuclear lamina; nuclear speckles; topologically associated domains; trans-chromosomal interaction.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests J.-H.S., P.Z., S.S.K., B.B., and X.Z. are inventors of a patent applied for by Harvard University related to the imaging technology. X.Z. is a co-founder and consultant of Vizgen, Inc.
Figures

Comment in
-
Tracing chromatin architecture.Nat Rev Genet. 2020 Nov;21(11):649. doi: 10.1038/s41576-020-00286-9. Nat Rev Genet. 2020. PMID: 32879440 No abstract available.
References
-
- Bickmore WA (2013). The spatial organization of the human genome. Annu. Rev. Genomics Hum. Genet 14, 67–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

