Therapy-Induced Evolution of Human Lung Cancer Revealed by Single-Cell RNA Sequencing
- PMID: 32822576
- PMCID: PMC7484178
- DOI: 10.1016/j.cell.2020.07.017
Therapy-Induced Evolution of Human Lung Cancer Revealed by Single-Cell RNA Sequencing
Abstract
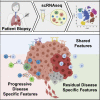
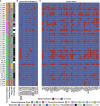
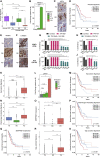
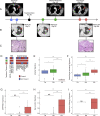
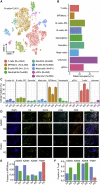
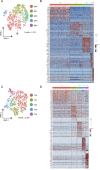
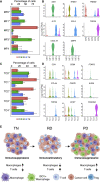
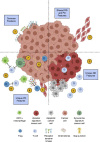
Lung cancer, the leading cause of cancer mortality, exhibits heterogeneity that enables adaptability, limits therapeutic success, and remains incompletely understood. Single-cell RNA sequencing (scRNA-seq) of metastatic lung cancer was performed using 49 clinical biopsies obtained from 30 patients before and during targeted therapy. Over 20,000 cancer and tumor microenvironment (TME) single-cell profiles exposed a rich and dynamic tumor ecosystem. scRNA-seq of cancer cells illuminated targetable oncogenes beyond those detected clinically. Cancer cells surviving therapy as residual disease (RD) expressed an alveolar-regenerative cell signature suggesting a therapy-induced primitive cell-state transition, whereas those present at on-therapy progressive disease (PD) upregulated kynurenine, plasminogen, and gap-junction pathways. Active T-lymphocytes and decreased macrophages were present at RD and immunosuppressive cell states characterized PD. Biological features revealed by scRNA-seq were biomarkers of clinical outcomes in independent cohorts. This study highlights how therapy-induced adaptation of the multi-cellular ecosystem of metastatic cancer shapes clinical outcomes.
Keywords: ALK; EGFR; lung cancer; single-cell RNA sequencing; targeted therapy.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests C.E.M., advisory board–Genentech; honoraria–Novartis, Guardant, Research Funding: Novartis, Revolution Medicines; J.K.R., advisory board: AstraZeneca, consulting: Takeda; E.L.S., employee – editorial contributor, Elsevier, PracticeUpdate.com; speakers fees: Takeda, Roche/Genentech, Physicians’ Education Resource, Medscape; Consultant: AbbVie; R.G.S., stock ownership in Celgene Corporation (Bristol-Myers Squibb); IP licensing: Newomics; S.B. consults with and/or receives research funding from Pfizer, Ideaya Biosciences and Revolution Medicines; M.G., research funding (to institution) for Celgene, Merck, Novartis, OncoMed, Roche; R.C.D., Advisory Board: Rain Therapeutics, Blueprint Medicines, Anchiano, Green Peptide, Genentech/Roche, Bayer, AstraZeneca; Intellectual Property Licensing: Rain Therapeutics, Foundation Medicine, Abbott Molecular, Black Diamond, Pearl River, Voronoi; Stock Ownership: Rain Therapeutics; J.W., Scientific Advisory Board member for Tenaya Therapeutics and Amgen. Founder and Consultant of KSQ Therapeutics and Maze Therapeutics. Venture Partner of 5AM Ventures; C.M.B., Consulting: Amgen, Foundation Medicine, Blueprint Medicines, Revolution Medicines; Research Funding: Novartis, AstraZeneca, Takeda; Institutional Research Funding: Mirati, Spectrum, MedImmune, Roche; T.G.B., Advisor to Novartis, Astrazeneca, Revolution Medicines, Array/Pfizer, Springworks, Strategia, Relay, Jazz, Rain and receives research funding from Novartis and Revolution Medicines and Strategia.
Figures






References
-
- Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A.J.R., Behjati S., Biankin A.V., Bignell G.R., Bolli N., Borg A., Børresen-Dale A.-L., Australian Pancreatic Cancer Genome Initiative. ICGC Breast Cancer Consortium. ICGC MMML-Seq Consortium. ICGC PedBrain Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

