Two Brain Pathways Initiate Distinct Forward Walking Programs in Drosophila
- PMID: 32822613
- PMCID: PMC9435592
- DOI: 10.1016/j.neuron.2020.07.032
Two Brain Pathways Initiate Distinct Forward Walking Programs in Drosophila
Abstract
An animal at rest or engaged in stationary behaviors can instantaneously initiate goal-directed walking. How descending brain inputs trigger rapid transitions from a non-walking state to an appropriate walking state is unclear. Here, we identify two neuronal types, P9 and BPN, in the Drosophila brain that, upon activation, initiate and maintain two distinct coordinated walking patterns. P9 drives forward walking with ipsilateral turning, receives inputs from central courtship-promoting neurons and visual projection neurons, and is necessary for a male to pursue a female during courtship. In contrast, BPN drives straight, forward walking and is not required during courtship. BPN is instead recruited during and required for fast, straight, forward walking bouts. Thus, this study reveals separate brain pathways for object-directed walking and fast, straight, forward walking, providing insight into how the brain initiates context-appropriate walking programs.
Keywords: Drosophila; behavior; courtship; descending neurons; locomotion; neural circuits; walking.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
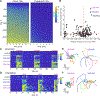

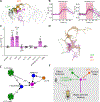

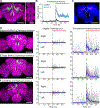


References
-
- Agrawal S, and Dickinson MH (2019). The effects of target contrast on Drosophila courtship. The Journal of Experimental Biology 222, jeb203414. - PubMed
-
- Agrawal S, Safarik S, and Dickinson M.(2014). The relative roles of vision and chemosensation in mate recognition of Drosophila melanogaster. J. Exp. Biol 217, 2796–2805. - PubMed
-
- Ampatzis K, Song J, Ausborn J, and El Manira A.(2014). Separate microcircuit modules of distinct v2a interneurons and motoneurons control the speed of locomotion. Neuron 83, 934–943. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

