Cytokines, JAK-STAT Signaling and Radiation-Induced DNA Repair in Solid Tumors: Novel Opportunities for Radiation Therapy
- PMID: 32822847
- PMCID: PMC8638530
- DOI: 10.1016/j.biocel.2020.105827
Cytokines, JAK-STAT Signaling and Radiation-Induced DNA Repair in Solid Tumors: Novel Opportunities for Radiation Therapy
Abstract
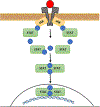
A number of solid tumors are treated with radiation therapy (RT) as a curative modality. At the same time, for certain types of cancers the applicable doses of RT are not high enough to result in a successful eradication of cancer cells. This is often caused by limited pharmacological tools and strategies to selectively sensitize tumors to RT while simultaneously sparing normal tissues from RT. We present an outline of a novel strategy for RT sensitization of solid tumors utilizing Jak inhibitors. Here, recently published pre-clinical data are reviewed which demonstrate the promising role of Jak inhibition in sensitization of tumors to RT. A wide number of currently approved Jak inhibitors for non-malignant conditions are summarized including Jak inhibitors currently in clinical development. Finally, intersection between Jak/Stat and the levels of serum cytokines are presented and discussed as they relate to susceptibility to RT.
Keywords: DNA repair; Jak; Solid tumors; Stat.
Copyright © 2020. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest
William A. Hall receives departmental research and travel support from Elekta AB Stockholm.
Figures
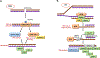

Similar articles
-
[Research Advances of JAK/STAT Signaling Pathway in Lung Cancer].Zhongguo Fei Ai Za Zhi. 2019 Jan 20;22(1):45-51. doi: 10.3779/j.issn.1009-3419.2019.01.09. Zhongguo Fei Ai Za Zhi. 2019. PMID: 30674393 Free PMC article. Review. Chinese.
-
Emerging therapeutic paradigms to target the dysregulated Janus kinase/signal transducer and activator of transcription pathway in hematological malignancies.Leuk Lymphoma. 2014 Sep;55(9):1968-79. doi: 10.3109/10428194.2013.863307. Epub 2014 Feb 17. Leuk Lymphoma. 2014. PMID: 24206094 Free PMC article. Review.
-
The role of JAK/STAT signaling pathway and its inhibitors in diseases.Int Immunopharmacol. 2020 Mar;80:106210. doi: 10.1016/j.intimp.2020.106210. Epub 2020 Jan 20. Int Immunopharmacol. 2020. PMID: 31972425 Review.
-
JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects.Drugs. 2017 Apr;77(5):521-546. doi: 10.1007/s40265-017-0701-9. Drugs. 2017. PMID: 28255960 Free PMC article. Review.
-
Targeting the interleukin-6/Jak/stat pathway in human malignancies.J Clin Oncol. 2012 Mar 20;30(9):1005-14. doi: 10.1200/JCO.2010.31.8907. Epub 2012 Feb 21. J Clin Oncol. 2012. PMID: 22355058 Free PMC article. Review.
Cited by
-
Prostate cancer stem cells and their targeted therapies.Front Cell Dev Biol. 2024 Aug 8;12:1410102. doi: 10.3389/fcell.2024.1410102. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39175878 Free PMC article. Review.
-
IFIT3 (interferon induced protein with tetratricopeptide repeats 3) modulates STAT1 expression in small extracellular vesicles.Biochem J. 2021 Nov 12;478(21):3905-3921. doi: 10.1042/BCJ20210580. Biochem J. 2021. PMID: 34622927 Free PMC article.
-
The DNA-dependent protein kinase catalytic subunit exacerbates endotoxemia-induced myocardial microvascular injury by disrupting the MOTS-c/JNK pathway and inducing profilin-mediated lamellipodia degradation.Theranostics. 2024 Feb 4;14(4):1561-1582. doi: 10.7150/thno.92650. eCollection 2024. Theranostics. 2024. PMID: 38389837 Free PMC article.
-
JAK/STAT of all trades: linking inflammation with cancer development, tumor progression and therapy resistance.Carcinogenesis. 2021 Dec 31;42(12):1411-1419. doi: 10.1093/carcin/bgab075. Carcinogenesis. 2021. PMID: 34415330 Free PMC article.
-
Impact of Different JAK Inhibitors and Methotrexate on Lymphocyte Proliferation and DNA Damage.J Clin Med. 2021 Apr 1;10(7):1431. doi: 10.3390/jcm10071431. J Clin Med. 2021. PMID: 33916057 Free PMC article.
References
-
- Ranjha L, Howard SM, Cejka P, 2018. Main steps in DNA double-strand break repair: an introduction to homologous recombination and related processes. Chromosoma 127, 187–214. - PubMed
-
- Roos WP, Thomas AD, Kaina B, 2016. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer 16, 20–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

