Drug screening model meets cancer organoid technology
- PMID: 32822897
- PMCID: PMC7451679
- DOI: 10.1016/j.tranon.2020.100840
Drug screening model meets cancer organoid technology
Abstract
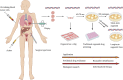
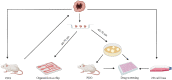
Tumor organoids inherit the genomic and molecular characteristics of the donor tumor, which not only bridge the gap between genome and phenotype but also circumvent the disadvantages such as genetic information change by using 2D cell lines and the mouse-specific tumor evolution in patient-derived xenograft (PDX). So, cancer organoid has been widely applied to preclinical drug evaluation, biomarker identification, biological research, and individualized therapy. Besides, cancer organoid can be preserved, resuscitated, passed infinitely, and mechanically cultured on a chip for drug screening; it has become one of the partial models for low/high-throughput drug screening in the preclinical trial in vitro. Therefore, this review presents the recent developments of tumor organoids for drug screening, which will introduce from four aspects, including the stability/credibility, types, application, deficiency and prospect of the tumor organoids model for drug screening.
Keywords: Cancer organoid; Drug screening; Precision therapy.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Harrison R.K. Phase II and phase III failures: 2013-2015. Nat. Rev. Drug Discov. 2016;15:817–818. - PubMed
-
- Tuveson D., Clevers H. Cancer modeling meets human organoid technology. Science. 2019;364:952–955. - PubMed
-
- Srivastava P., Kumar M., Nayak P. 2016. Role of Patient Derived Cell Lines and Xenograft in Cancer Research. The Pharmstudent; p. 27.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous