Opening up the Toolbox: Synthesis and Mechanisms of Phosphoramidates
- PMID: 32823507
- PMCID: PMC7463754
- DOI: 10.3390/molecules25163684
Opening up the Toolbox: Synthesis and Mechanisms of Phosphoramidates
Abstract
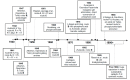
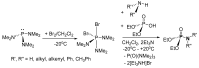
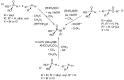
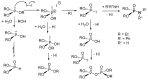
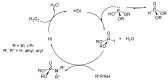
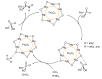
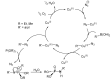
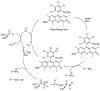
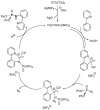
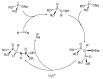
This review covers the main synthetic routes to and the corresponding mechanisms of phosphoramidate formation. The synthetic routes can be separated into six categories: salt elimination, oxidative cross-coupling, azide, reduction, hydrophosphinylation, and phosphoramidate-aldehyde-dienophile (PAD). Examples of some important compounds synthesized through these routes are provided. As an important class of organophosphorus compounds, the applications of phosphoramidate compounds, are also briefly introduced.
Keywords: applications; mechanism; phosphoramidate; synthetic routes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
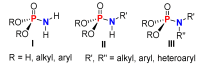


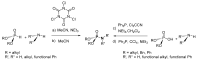
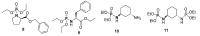

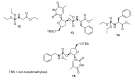



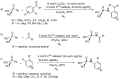
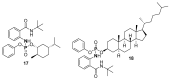




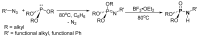




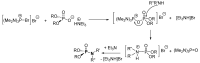


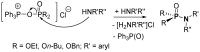
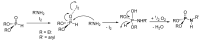







References
-
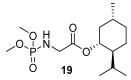
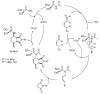
- Chabour I., Nájera C., Sansano J.M. Diastereoselective multicomponent phosphoramidate-aldehyde-dienophile (PAD) process for the synthesis of polysubstituted cyclohex-2-enyl-amine derivatives. Tetrahedron. 2020;76:130801. doi: 10.1016/j.tet.2019.130801. - DOI
-
- Sekine M., Moriguchi T., Wada T., Seio K. Total Synthesis of Agrocin 84 and Phosmidosine as Naturally Occurring Nucleotidic Antibiotics Having P-N Bond Linkages. J. Syn. Org. Chem. Jpn. 2001;59:1109–1120. doi: 10.5059/yukigoseikyokaishi.59.1109. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

