Low-Level Ionizing Radiation Induces Selective Killing of HIV-1-Infected Cells with Reversal of Cytokine Induction Using mTOR Inhibitors
- PMID: 32823598
- PMCID: PMC7472203
- DOI: 10.3390/v12080885
Low-Level Ionizing Radiation Induces Selective Killing of HIV-1-Infected Cells with Reversal of Cytokine Induction Using mTOR Inhibitors
Abstract
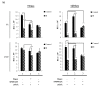
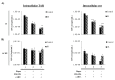
HIV-1 infects 39.5 million people worldwide, and cART is effective in preventing viral spread by reducing HIV-1 plasma viral loads to undetectable levels. However, viral reservoirs persist by mechanisms, including the inhibition of autophagy by HIV-1 proteins (i.e., Nef and Tat). HIV-1 reservoirs can be targeted by the "shock and kill" strategy, which utilizes latency-reversing agents (LRAs) to activate latent proviruses and immunotarget the virus-producing cells. Yet, limitations include reduced LRA permeability across anatomical barriers and immune hyper-activation. Ionizing radiation (IR) induces effective viral activation across anatomical barriers. Like other LRAs, IR may cause inflammation and modulate the secretion of extracellular vesicles (EVs). We and others have shown that cells may secrete cytokines and viral proteins in EVs and, therefore, LRAs may contribute to inflammatory EVs. In the present study, we mitigated the effects of IR-induced inflammatory EVs (i.e., TNF-α), through the use of mTOR inhibitors (mTORi; Rapamycin and INK128). Further, mTORi were found to enhance the selective killing of HIV-1-infected myeloid and T-cell reservoirs at the exclusion of uninfected cells, potentially via inhibition of viral transcription/translation and induction of autophagy. Collectively, the proposed regimen using cART, IR, and mTORi presents a novel approach allowing for the targeting of viral reservoirs, prevention of immune hyper-activation, and selectively killing latently infected HIV-1 cells.
Keywords: HIV-1; HIV-1 therapy; Ionizing radiation; autophagy; cell death; extracellular vesicles; inflammation; latency reversal; mTOR inhibition; shock and kill.
Conflict of interest statement
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Figures







References
-
- Global AIDS Update 2019. UNAIDS; Geneva, Switzerland: 2019. p. 316.
-
- Hatano H., Jain V., Hunt P.W., Lee T.H., Sinclair E., Do T.D., Hoh R., Martin J.N., McCune J.M., Hecht F., et al. Cell-based measures of viral persistence are Associated with immune activation and programmed cell death protein 1 (PD-1)–expressing CD4+ T cells. J. Infect. Dis. 2013;208:50–56. doi: 10.1093/infdis/jis630. - DOI - PMC - PubMed
-
- Imamichi H., Dewar R.L., Adelsberger J.W., Rehm C.A., O’Doherty U., Paxinos E.E., Fauci A.S., Lane H.C. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. PNAS. 2016;113:8783–8788. doi: 10.1073/pnas.1609057113. - DOI - PMC - PubMed
-
- Iordanskiy S., Van Duyne R., Sampey G.C., Woodson C.M., Fry K., Saifuddin M., Guo J., Wu Y., Romerio F., Kashanchi F., et al. Therapeutic doses of irradiation activate viral transcription and induce apoptosis in HIV-1 infected cells. Virology. 2015;485:1–15. doi: 10.1016/j.virol.2015.06.021. - DOI - PMC - PubMed
-
- Fields J.A., Spencer B., Swinton M., Qvale E.M., Marquine M.J., Alexeeva A., Gough S., Soontornniyomkij B., Valera E., Masliah E., et al. Alterations in brain TREM2 and Amyloid-β levels are associated with neurocognitive impairment in HIV-infected persons on antiretroviral therapy. J. Neurochem. 2018 doi: 10.1111/jnc.14582. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

