EphrinA1-Fc Attenuates Ventricular Remodeling and Dysfunction in Chronically Nonreperfused WT but not EphA2-R-M mice
- PMID: 32823610
- PMCID: PMC7461052
- DOI: 10.3390/ijms21165811
EphrinA1-Fc Attenuates Ventricular Remodeling and Dysfunction in Chronically Nonreperfused WT but not EphA2-R-M mice
Abstract
Background: EphrinA1-Fc abolishes acute I/R injury and attenuates nonreperfused cardiac injury 4 days after permanent occlusion in mice. The goal of this study was to assess the capacity of a single intramyocardial administration of ephrinA1-Fc at the time of coronary artery ligation, to determine the degree to which early salvage effects translate to reduced adverse remodeling after 4 weeks of nonreperfused myocardial infarction (MI) in wild-type B6 and EphA2-R-M (EphA2 receptor null) mice.
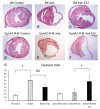
Methods: At 4 weeks post-MI, echocardiography, histologic and immunohistochemical analyses of B6 mouse hearts were performed. Primary mouse cardiac fibroblasts (FBs) isolated from B6 mice cultured in the presence of low and high dose ephrinA1-Fc, both with and without pro-fibrotic TGF-β stimulation and Western blots, were probed for relative expression of remodeling proteins MMP-2, MMP-9 and TIMP-1, in addition to DDR2 and (p)SMAD2/3/totalSMAD2/3.
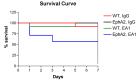
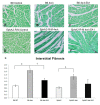
Results: EphrinA1-Fc preserved a significant degree of contractile function, decreased adverse left ventricular remodeling, attenuated excessive compensatory hypertrophy, and decreased interstitial fibrosis in wild-type (WT) B6 mouse hearts. In contrast, most of these parameters were poorer in ephrinA1-Fc-treated EphA2-R-M mice. Of note, fibrosis was proportionately decreased, implying that other EphA receptor(s) are more important in regulating the pro-fibrotic response. Primary FBs showed disparate alteration of MMP-2, MMP-9 and TIMP-1, as well as DDR2 and p-SMAD2/3/totalSMAD2/3, which indicates that matrix remodeling and cardiac fibrosis in the injured heart are influenced by ephrinA1-Fc.
Conclusion: This study demonstrates the capacity of a single administration of ephrinA1-Fc at the onset of injury to attenuate long-term nonreperfused post-MI ventricular remodeling that results in progressive heart failure, and the important role of EphA2 in mitigating the deleterious effects.
Keywords: EphA2; ephrinA1; fibrosis; myocardial infarction; remodeling.
Conflict of interest statement
All authors declare that there are no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

