The Hippo Pathway Effector YAP1 Regulates Intestinal Epithelial Cell Differentiation
- PMID: 32823612
- PMCID: PMC7463744
- DOI: 10.3390/cells9081895
The Hippo Pathway Effector YAP1 Regulates Intestinal Epithelial Cell Differentiation
Abstract
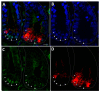
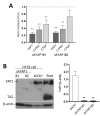
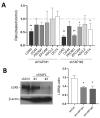
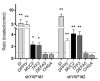
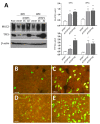
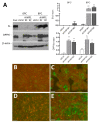
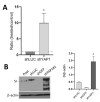
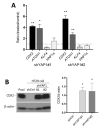
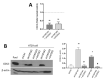
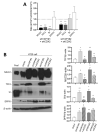
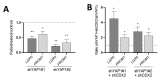
The human intestine is covered by epithelium, which is continuously replaced by new cells provided by stem cells located at the bottom of the glands. The maintenance of intestinal stem cells is supported by a niche which is composed of several signaling proteins including the Hippo pathway effectors YAP1/TAZ. The role of YAP1/TAZ in cell proliferation and regeneration is well documented but their involvement on the differentiation of intestinal epithelial cells is unclear. In the present study, the role of YAP1/TAZ on the differentiation of intestinal epithelial cells was investigated using the HT29 cell line, the only multipotent intestinal cell line available, with a combination of knockdown approaches. The expression of intestinal differentiation cell markers was tested by qPCR, Western blot, indirect immunofluorescence and electron microscopy analyses. The results show that TAZ is not expressed while the abolition of YAP1 expression led to a sharp increase in goblet and absorptive cell differentiation and reduction of some stem cell markers. Further studies using double knockdown experiments revealed that most of these effects resulting from YAP1 abolition are mediated by CDX2, a key intestinal cell transcription factor. In conclusion, our results indicate that YAP1/TAZ negatively regulate the differentiation of intestinal epithelial cells through the inhibition of CDX2 expression.
Keywords: CDX2; Hippo pathway; TAZ; YAP1; absorptive cells; differentiation; goblet cell; intestinal cell; stem cell.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, nor in the decision to publish the results.
Figures

Similar articles
-
Src family kinases inhibit differentiation of intestinal epithelial cells through the Hippo effector YAP1.Biol Open. 2021 Nov 15;10(11):bio058904. doi: 10.1242/bio.058904. Epub 2021 Nov 15. Biol Open. 2021. PMID: 34693980 Free PMC article.
-
Differential influence of YAP1 and TAZ on differentiation of intestinal epithelial cell: A review.Anat Rec (Hoboken). 2023 May;306(5):1054-1061. doi: 10.1002/ar.24996. Epub 2022 Jun 1. Anat Rec (Hoboken). 2023. PMID: 35648375 Review.
-
Effects of the hippo signaling pathway in human gastric cancer.Asian Pac J Cancer Prev. 2013;14(9):5199-205. doi: 10.7314/apjcp.2013.14.9.5199. Asian Pac J Cancer Prev. 2013. PMID: 24175801
-
Hippo-YAP1 signaling pathway and severe preeclampsia (sPE) in the Chinese population.Pregnancy Hypertens. 2020 Jan;19:1-10. doi: 10.1016/j.preghy.2019.11.002. Epub 2019 Dec 13. Pregnancy Hypertens. 2020. PMID: 31841877
-
The Hippo Pathway as Drug Targets in Cancer Therapy and Regenerative Medicine.Curr Drug Targets. 2017;18(4):447-454. doi: 10.2174/1389450117666160112115641. Curr Drug Targets. 2017. PMID: 26758663 Review.
Cited by
-
Src family kinases inhibit differentiation of intestinal epithelial cells through the Hippo effector YAP1.Biol Open. 2021 Nov 15;10(11):bio058904. doi: 10.1242/bio.058904. Epub 2021 Nov 15. Biol Open. 2021. PMID: 34693980 Free PMC article.
-
Peristalsis-Associated Mechanotransduction Drives Malignant Progression of Colorectal Cancer.Cell Mol Bioeng. 2023 Aug 11;16(4):261-281. doi: 10.1007/s12195-023-00776-w. eCollection 2023 Aug. Cell Mol Bioeng. 2023. PMID: 37811008 Free PMC article.
-
The study of an anoikis-related signature to predict glioma prognosis and immune infiltration.J Cancer Res Clin Oncol. 2023 Nov;149(14):12659-12676. doi: 10.1007/s00432-023-05138-7. Epub 2023 Jul 14. J Cancer Res Clin Oncol. 2023. PMID: 37450027 Free PMC article.
-
JAM-A signals through the Hippo pathway to regulate intestinal epithelial proliferation.iScience. 2022 Apr 27;25(5):104316. doi: 10.1016/j.isci.2022.104316. eCollection 2022 May 20. iScience. 2022. PMID: 35602956 Free PMC article.
-
Primary Cilium Identifies a Quiescent Cell Population in the Human Intestinal Crypt.Cells. 2023 Mar 31;12(7):1059. doi: 10.3390/cells12071059. Cells. 2023. PMID: 37048132 Free PMC article.
References
-
- Fallah S., Sénicourt B., Beaulieu J.-F. Proliferation in the Gastrointestinal Epithelium. In: Kuipers E.J., editor. Encyclopedia of Gastroenterology. 2nd ed. Academic Press; Oxford, UK: 2020. pp. 304–310.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

