The Ascidian-Derived Metabolites with Antimicrobial Properties
- PMID: 32823633
- PMCID: PMC7460354
- DOI: 10.3390/antibiotics9080510
The Ascidian-Derived Metabolites with Antimicrobial Properties
Abstract
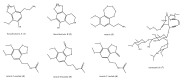
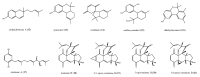
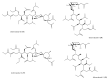
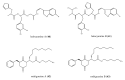
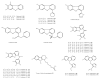
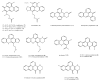
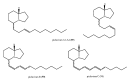
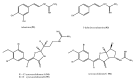
Among the sub-phylum of Tunicate, ascidians represent the most abundant class of marine invertebrates, with 3000 species by heterogeneous habitat, that is, from shallow water to deep sea, already reported. The chemistry of these sessile filter-feeding organisms is an attractive reservoir of varied and peculiar bioactive compounds. Most secondary metabolites isolated from ascidians stand out for their potential as putative therapeutic agents in the treatment of several illnesses like microbial infections. In this review, we present and discuss the antibacterial activity shown by the main groups of ascidian-derived products, such as sulfur-containing compounds, meroterpenes, alkaloids, peptides, furanones, and their derivatives. Moreover, the direct evidence of a symbiotic association between marine ascidians and microorganisms shed light on the real producers of many extremely potent marine natural compounds. Hence, we also report the antibacterial potential, joined to antifungal and antiviral activity, of metabolites isolated from ascidian-associate microorganisms by culture-dependent methods.
Keywords: antibacterial; antimicrobial; antiviral; ascidian; ascidian-associated microorganisms; marine natural products.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



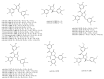

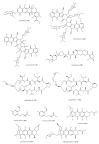
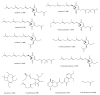
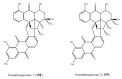
References
-
- WHO No Time to Wait: Securing the Future from Drug-Resistant Infections. [(accessed on 30 April 2019)];2019 Apr; Available online: https://www.who.int/antimicrobial-resistance/interagency-coordination-gr...
-
- Silva O.N., de La Fuente-Núñez C., Haney E.F., Fensterseifer I.C., Ribeiro S.M., Porto W.F., Brown P., Faria-Junior C., Rezende T.M., Moreno S.E., et al. An Anti-Infective Synthetic Peptide with Dual Antimicrobial and Immunomodulatory Activities. Sci. Rep. 2016;6:35465. doi: 10.1038/srep35465. - DOI - PMC - PubMed
-
- Millero F.J. Chemical Oceanography. 4th ed. CRC Press Taylor & Francis Group; Boca Raton, FL, USA: 2013. Descriptive Oceanography.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

