Gut-Pancreas-Liver Axis as a Target for Treatment of NAFLD/NASH
- PMID: 32823659
- PMCID: PMC7461212
- DOI: 10.3390/ijms21165820
Gut-Pancreas-Liver Axis as a Target for Treatment of NAFLD/NASH
Abstract
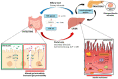
Non-alcoholic fatty liver disease (NAFLD) represents the most common form of chronic liver disease worldwide. Due to its association with obesity and diabetes and the fall in hepatitis C virus morbidity, cirrhosis in NAFLD is becoming the most frequent indication to liver transplantation, but the pathogenetic mechanisms are still not completely understood. The so-called gut-liver axis has gained enormous interest when data showed that its alteration can lead to NAFLD development and might favor the occurrence of non-alcoholic steatohepatitis (NASH). Moreover, several therapeutic approaches targeting the gut-pancreas-liver axis, e.g., incretins, showed promising results in NASH treatment. In this review, we describe the role of incretin hormones in NAFLD/NASH pathogenesis and treatment and how metagenomic/metabolomic alterations in the gut microbiota can lead to NASH in the presence of gut barrier modifications favoring the passage of bacteria or bacterial products in the portal circulation, i.e., bacterial translocation.
Keywords: glucose metabolism; gut-pancreas-liver axis; incretins; lipid metabolism; non-alcoholic fatty liver disease; non-alcoholic steatohepatitis; type-2 diabetes.
Conflict of interest statement
The authors have no potential conflict of interest relevant to this article.
Figures


References
-
- Bedossa P., Tordjman J., Aron-Wisnewsky J., Poitou C., Oppert J.M., Torcivia A., Bouillot J.L., Paradis V., Ratziu V., Clement K. Systematic review of bariatric surgery liver biopsies clarifies the natural history of liver disease in patients with severe obesity. Gut. 2017;66:1688–1696. doi: 10.1136/gutjnl-2016-312238. - DOI - PubMed
-
- Souto K.P., Meinhardt N.G., Ramos M.J., Ulbrich-Kulkzynski J.M., Stein A.T., Damin D.C. Nonalcoholic fatty liver disease in patients with different baseline glucose status undergoing bariatric surgery: Analysis of intraoperative liver biopsies and literature review. Surg. Obes. Relat. Dis. 2018;14:66–73. doi: 10.1016/j.soard.2017.09.527. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

