Mechanisms of Phase Transformation and Creating Mechanical Strength in a Sustainable Calcium Carbonate Cement
- PMID: 32823671
- PMCID: PMC7476014
- DOI: 10.3390/ma13163582
Mechanisms of Phase Transformation and Creating Mechanical Strength in a Sustainable Calcium Carbonate Cement
Abstract
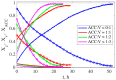
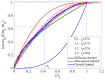
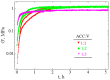
Calcium carbonate cements have been synthesized by mixing amorphous calcium carbonate and vaterite powders with water to form a cement paste and study how mechanical strength is created during the setting reaction. In-situ X-ray diffraction (XRD) was used to monitor the transformation of amorphous calcium carbonate (ACC) and vaterite phases into calcite and a rotational rheometer was used to monitor the strength evolution. There are two characteristic timescales of the strengthening of the cement paste. The short timescale of the order 1 h is controlled by smoothening of the vaterite grains, allowing closer and therefore adhesive contacts between the grains. The long timescale of the order 10-50 h is controlled by the phase transformation of vaterite into calcite. This transformation is, unlike in previous studies using stirred reactors, found to be mainly controlled by diffusion in the liquid phase. The evolution of shear strength with solid volume fraction is best explained by a fractal model of the paste structure.
Keywords: calcium carbonate; cement; colloidal suspension; hardening; phase transformation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Gartner E.M., Macphee D.E. A physico-chemical basis for novel cementitious binders. Cem. Concr. Res. 2011;41:736–749. doi: 10.1016/j.cemconres.2011.03.006. - DOI
-
- Provis J.L., Deventer J.S.J.V. Alkali Activated Materials. Springer; Dordrecht, The Netherlands: 2014.
-
- Zhang T., Cheeseman C.R., Vandeperre L.J. Development of low pH cement systems forming magnesium silicate hydrate (M-S-H) Cem. Concr. Res. 2011;41:439–442. doi: 10.1016/j.cemconres.2011.01.016. - DOI
-
- Justnes H. Alternative Low-CO2 “Green” Clinkering Processes. Rev. Mineral. Geochem. 2012;74:83–99. doi: 10.2138/rmg.2012.74.2. - DOI
-
- Fontaine M.-L., Combes C., Sillam T., Dechambre G., Rey C. New Calcium Carbonate-Based Cements for Bone Reconstruction. Key Eng. Mater. 2005;284–286:105–108. doi: 10.4028/www.scientific.net/KEM.284-286.105. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

