Involvement of JNK1 in Neuronal Polarization During Brain Development
- PMID: 32823764
- PMCID: PMC7466125
- DOI: 10.3390/cells9081897
Involvement of JNK1 in Neuronal Polarization During Brain Development
Abstract
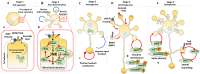
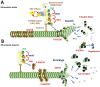
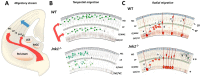
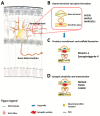
The c-Jun N-terminal Kinases (JNKs) are a group of regulatory elements responsible for the control of a wide array of functions within the cell. In the central nervous system (CNS), JNKs are involved in neuronal polarization, starting from the cell division of neural stem cells and ending with their final positioning when migrating and maturing. This review will focus mostly on isoform JNK1, the foremost contributor of total JNK activity in the CNS. Throughout the text, research from multiple groups will be summarized and discussed in order to describe the involvement of the JNKs in the different steps of neuronal polarization. The data presented support the idea that isoform JNK1 is highly relevant to the regulation of many of the processes that occur in neuronal development in the CNS.
Keywords: DCX; JNK1; MAP1B; NMDAR; SCG10; WDR62; asymmetric division; migration; synaptogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
JNK1 phosphorylation of SCG10 determines microtubule dynamics and axodendritic length.J Cell Biol. 2006 Apr 24;173(2):265-77. doi: 10.1083/jcb.200511055. Epub 2006 Apr 17. J Cell Biol. 2006. PMID: 16618812 Free PMC article.
-
c-Jun N-terminal protein kinase (JNK) 2/3 is specifically activated by stress, mediating c-Jun activation, in the presence of constitutive JNK1 activity in cerebellar neurons.J Neurosci. 2002 Jun 1;22(11):4335-45. doi: 10.1523/JNEUROSCI.22-11-04335.2002. J Neurosci. 2002. PMID: 12040039 Free PMC article.
-
Neuroprotective Effects of the Absence of JNK1 or JNK3 Isoforms on Kainic Acid-Induced Temporal Lobe Epilepsy-Like Symptoms.Mol Neurobiol. 2018 May;55(5):4437-4452. doi: 10.1007/s12035-017-0669-1. Epub 2017 Jun 29. Mol Neurobiol. 2018. PMID: 28664455
-
From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance.IUBMB Life. 2005 Apr-May;57(4-5):283-95. doi: 10.1080/15216540500097111. IUBMB Life. 2005. PMID: 16036612 Review.
-
JNK and p38 stresskinases--degenerative effectors of signal-transduction-cascades in the nervous system.Prog Neurobiol. 2000 May;61(1):45-60. doi: 10.1016/s0301-0082(99)00042-8. Prog Neurobiol. 2000. PMID: 10759064 Review.
Cited by
-
Convergent Canonical Pathways in Autism Spectrum Disorder from Proteomic, Transcriptomic and DNA Methylation Data.Int J Mol Sci. 2021 Oct 5;22(19):10757. doi: 10.3390/ijms221910757. Int J Mol Sci. 2021. PMID: 34639097 Free PMC article. Review.
-
The role of JNK signaling pathway in organ fibrosis.J Adv Res. 2025 Aug;74:207-223. doi: 10.1016/j.jare.2024.09.029. Epub 2024 Oct 2. J Adv Res. 2025. PMID: 39366483 Free PMC article. Review.
-
Integrative analysis identifies IL-6/JUN/MMP-9 pathway destroyed blood-brain-barrier in autism mice via machine learning and bioinformatic analysis.Transl Psychiatry. 2025 Jul 11;15(1):239. doi: 10.1038/s41398-025-03452-x. Transl Psychiatry. 2025. PMID: 40645949 Free PMC article.
-
Inflammatory signaling pathways in the treatment of Alzheimer's disease with inhibitors, natural products and metabolites (Review).Int J Mol Med. 2023 Nov;52(5):111. doi: 10.3892/ijmm.2023.5314. Epub 2023 Oct 6. Int J Mol Med. 2023. PMID: 37800614 Free PMC article. Review.
-
The Role of the Dysregulated JNK Signaling Pathway in the Pathogenesis of Human Diseases and Its Potential Therapeutic Strategies: A Comprehensive Review.Biomolecules. 2024 Feb 19;14(2):243. doi: 10.3390/biom14020243. Biomolecules. 2024. PMID: 38397480 Free PMC article. Review.
References
-
- Sabapathy K. Role of the JNK Pathway in Human Diseases. Elsevier; Amsterdam, The Netherlands: 2012. pp. 145–169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

